JAMA Network
COVID-19 Update January 23, 2021
These articles on COVID-19 were published across the JAMA Network in the last week.
JAMA Network
COVID-19 Update January 23, 2021
These articles on COVID-19 were published across the JAMA Network in the last week.
JBI Evidence Synthesis
January 2021 – Volume 19 – Issue 1
https://journals.lww.com/jbisrir/Pages/currenttoc.aspx
SYSTEMATIC REVIEW PROTOCOLS
Point-of-care testing for sexually transmitted infections in low- and middle-income countries: a scoping review protocol
Martin, Kevin; Roper, Tom; Vera, Jaime H.
JBI Evidence Synthesis. 19(1):155-162, January 2021.
Journal of Pharmaceutical Policy and Practice
https://joppp.biomedcentral.com/
[Accessed 23 Jan 2021]
Making the investment case for national regulatory authorities
Well-functioning national regulatory authorities (NRAs) ensure access to safe, effective, quality-assured, and affordable medical products. However, the benefits of their work are often unseen and difficult to…
Authors: Gloria Twesigye, Tamara Hafner and Javier Guzman
Citation: Journal of Pharmaceutical Policy and Practice 2021 14:16
Content type: Commentary
Published on: 21 January 2021
Journal of Pharmaceutical Policy and Practice
https://joppp.biomedcentral.com/
[Accessed 23 Jan 2021]
Drug supply situation in Rwanda during COVID-19: issues, efforts and challenges
COVID-19 is a threat to health systems around the world and Rwanda is not an exception. The impact of the pandemic is far-reaching and access to health commodities is not spared. Proper drug supply is critical…
Authors: Theogene Uwizeyimana, Hashim Talib Hashim, Jean Damascene Kabakambira, Jean Claude Mujyarugamba, Jackson Dushime, Blaise Ntacyabukura, Remy Ndayizeye, Yusuff Adebayo Adebisi and Don Eliseo Lucero-Prisno III
Citation: Journal of Pharmaceutical Policy and Practice 2021 14:12
Content type: Commentary
Published on: 20 January 2021
The Lancet
Jan 23, 2021 Volume 397 Number 10271 p253-346, e3-e5
https://www.thelancet.com/journals/lancet/issue/current
Editorial
COVID-19: the intersection of education and health
The Lancet
What lessons does the COVID-19 syndemic offer when considering the convergence between health and education? The International Day of Education, on Jan 24, provides an opportunity to reflect on the weaknesses of the education system before COVID-19, and on the impact of school closures and education disruptions on children and adolescents. Since March, 2020, more than 1·5 billion students worldwide—an unprecedented number—have been affected by school or university closures. The implications of these closures are enormous. In addition to the loss of learning, a lack of access to school means that many children lose protection from hazards such as domestic violence and child abuse, others lose access to the only nutritious meal of their day, and many will miss immunisations that are often given at school. Furthermore, school closures deprive children and adolescents of social and emotional experiences essential for their development and wellbeing.
Adolescents are particularly affected by both closures and by distance learning in higher education. In the short term, some students are leaving school to find work earlier than they might otherwise have; others might be experiencing mental health problems such as loneliness and anxiety. In the long term, there is a danger that hard-won progress in secondary school attendance in low-income and middle-income countries will be reversed. And it is not only schools that shape education. Cultural events, sport, and religion have been disrupted, in many countries for almost a year. Under lockdowns, children who have not yet reached school age have been forced to remain at home, and low levels of stimulation during a child’s early years are likely to have far-reaching consequences for their development. Health and education are bidirectionally linked: a good-quality education is an investment for health, and health is essential for effective learning. These disruptions to education, and the subsequent widening of inequalities in learning, will adversely affect the health of this generation and their children.
The disproportionate effect of school closures on girls and poorer students is especially concerning—millions of children are predicted to drop out of school (the humanitarian analysis organisation ACAPS says 24 million; Save the Children estimates 9·7 million). Many educational institutions have re-established their programmes online to mitigate short-term interruptions in learning. However, the effects of a digital divide and intangible losses of cognitive and social skills cannot be easily repaired. The economic crisis is pushing poor households into greater poverty, with families turning to early marriage as an alternative form of income. This predicament further perpetuates intergenerational poverty and inequality. Education is the only ladder out of poverty for many children and adolescents, and it is crucial to empower girls to economic independence and resist violation of their rights.
Education systems will be most beneficial when they provide more than a curriculum in science, maths, languages, and other academic subjects. Programmes that better support the cognitive and behavioural skills of children—self-reliance, decision making, anxiety management, communication, and assertiveness—will enable them to thrive. Traditional educational skills need to be expanded to encompass training in sexual and reproductive health and rights, child nutrition, and mental health.
This conceptual change in the value of education needs to start at the national level with revitalised education programmes. There is evidence that holistic approaches to education that value health and wellbeing can be effective. But their success is dependent on the political will to implement and support them. For example, the Health Promoting Schools approach developed by WHO values schools as social communities inclusive of students, teachers, and families; however, WHO reports that few countries have successfully implemented it at scale. This approach is aligned with the Sustainable Development Goals (SDGs) for health (SGD3) and quality education (SDG4), which explicitly acknowledge the linkage between health and education. Yet, the two sectors remain distant; arguments over whether to close schools to prevent infection can even imply that they are in opposition. This disconnect needs to be remedied. Closer cooperation would revitalise not only education, but also child and adolescent health.
Nature
Volume 589 Issue 7842, 21 January 2021
http://www.nature.com/nature/current_issue.html
News & Views | 16 December 2020
Precise mapping reveals gaps in global measles vaccination coverage
Precise maps of routine first-dose measles vaccinations show slowing progress around the world between 2010 and 2019, and large gaps in coverage in many places. Many countries are unlikely to achieve global 2020 coverage targets.
C. Edson Utazi & Andrew J. Tatem
Nature
Volume 589 Issue 7842, 21 January 2021
http://www.nature.com/nature/current_issue.html
Article | 16 December 2020 | Open Access
Mapping routine measles vaccination in low- and middle-income countries
Alyssa N. Sbarra, Sam Rolfe[…] & Jonathan F. Mosser
Abstract
The safe, highly effective measles vaccine has been recommended globally since 1974, yet in 2017 there were more than 17 million cases of measles and 83,400 deaths in children under 5 years old, and more than 99% of both occurred in low- and middle-income countries (LMICs)1,2,3,4. Globally comparable, annual, local estimates of routine first-dose measles-containing vaccine (MCV1) coverage are critical for understanding geographically precise immunity patterns, progress towards the targets of the Global Vaccine Action Plan (GVAP), and high-risk areas amid disruptions to vaccination programmes caused by coronavirus disease 2019 (COVID-19)5,6,7,8. Here we generated annual estimates of routine childhood MCV1 coverage at 5 × 5-km2 pixel and second administrative levels from 2000 to 2019 in 101 LMICs, quantified geographical inequality and assessed vaccination status by geographical remoteness. After widespread MCV1 gains from 2000 to 2010, coverage regressed in more than half of the districts between 2010 and 2019, leaving many LMICs far from the GVAP goal of 80% coverage in all districts by 2019. MCV1 coverage was lower in rural than in urban locations, although a larger proportion of unvaccinated children overall lived in urban locations; strategies to provide essential vaccination services should address both geographical contexts. These results provide a tool for decision-makers to strengthen routine MCV1 immunization programmes and provide equitable disease protection for all children.
New England Journal of Medicine
January 21, 2021 Vol. 384 No. 3
http://www.nejm.org/toc/nejm/medical-journal
Original Articles
Post-Transcriptional Genetic Silencing of BCL11A to Treat Sickle Cell Disease – E.B. Esrick and Others
New England Journal of Medicine
January 21, 2021 Vol. 384 No. 3
http://www.nejm.org/toc/nejm/medical-journal
PLoS One
http://www.plosone.org/
[Accessed 23 Jan 2021]
Measles epidemic in pediatric population in Greece during 2017–2018: Epidemiological, clinical characteristics and outcomes
Maria Gianniki, Tania Siahanidou, Evanthia Botsa, Athanasios Michos
Research Article | published 20 Jan 2021 PLOS ONE
https://doi.org/10.1371/journal.pone.0245512
PNAS – Proceedings of the National Academy of Sciences of the United States of America
January 19, 2021 118 (3)
https://www.pnas.org/content/118/2
Opinion
Opinion: Standardizing gene product nomenclature—a call to action
Kenji Fujiyoshi, Elspeth A. Bruford, Pawel Mroz, Cynthe L. Sims, Timothy J. O’Leary, Anthony W. I. Lo, Neng Chen, Nimesh R. Patel, Keyur Pravinchandra Patel, Barbara Seliger, Mingyang Song, Federico A. Monzon, Alexis B. Carter, Margaret L. Gulley, Susan M. Mockus, Thuy L. Phung, Harriet Feilotter, Heather E. Williams, and Shuji Ogino
PNAS January 19, 2021 118 (3) e2025207118; https://doi.org/10.1073/pnas.2025207118
The current lack of a standardized nomenclature system for gene products (e.g., proteins) has resulted in a haphazard counterproductive system of labeling. Different names are often used for the same gene product; the same name is sometimes used for unrelated gene products. Such ambiguity causes not only potential harm to patients, whose treatments increasingly rely on laboratory tests for multiple gene products, but also miscommunication and inefficiency, both of which hinder progress of broad scientific fields. To mitigate this confusion, we recommend standardizing human protein nomenclature through the use of a Human Genome Organisation (HUGO) Gene Nomenclature Committee (HGNC) gene symbol accompanied by its unique HGNC ID. We call for action across all biomedical communities and scientific and medical journals to standardize nomenclature of gene products using HGNC gene symbols to enhance accuracy in scientific and public communication.
PNAS – Proceedings of the National Academy of Sciences of the United States of America
January 19, 2021 118 (3)
https://www.pnas.org/content/118/2
Perspective Open Access
Vaccinology in the post−COVID-19 era
Rino Rappuoli, Ennio De Gregorio, Giuseppe Del Giudice, Sanjay Phogat, Simone Pecetta, Mariagrazia Pizza, and Emmanuel Hanon
PNAS January 19, 2021 118 (3) e2020368118; https://doi.org/10.1073/pnas.2020368118
Abstract
The COVID-19 pandemic is a shocking reminder of how our world would look in the absence of vaccination. Fortunately, new technologies, the pace of understanding new and existing pathogens, and the increased knowledge of the immune system allow us today to develop vaccines at an unprecedented speed. Some of the vaccine technologies that are fast-tracked by the urgency of COVID-19 may also be the answer for other health priorities, such as antimicrobial resistance, chronic infections, and cancer, that the post-COVID-19 world will urgently need to face. This perspective analyzes the way COVID-19 is transforming vaccinology and the opportunities for vaccines to have an increasingly important role in health and well-being.
Reproductive Health
http://www.reproductive-health-journal.com/content
[Accessed 23 Jan 2021]
The impact of the COVID-19 pandemic on maternal and perinatal health: a scoping review
The Covid-19 pandemic affects maternal health both directly and indirectly, and direct and indirect effects are intertwined. To provide a comprehensive overview on this broad topic in a rapid format behooving …
Authors: Bethany Kotlar, Emily Gerson, Sophia Petrillo, Ana Langer and Henning Tiemeier
Citation: Reproductive Health 2021 18:10
Content type: Review
Published on: 18 January 2021
Revista Panamericana de Salud Pública/Pan American Journal of Public Health (RPSP/PAJPH)
https://www.paho.org/journal/en
22 Jan 2021
Just societies: A new vision for health equity in the Americas after COVID-19
Editorial | English |
[Extract]
The significant challenges to equity in health in the Region of the Americas, as detailed in the report of the Pan American Health Organization Independent Commission on Equity and Health Inequalities in the Americas (1), gave original impetus to this Special Issue on Equity in Health by the Pan American Journal of Public Health. The report, Just Societies: Health Equity and Dignified Lives, analyzed a vast body of evidence that indicated the overwhelming inequalities in the Region that relate to three factors: structural drivers, conditions of daily life, and governance for health equity (taking action).
Highlighting the continued realities of the interrelationship between social and health inequities in the Americas is by no means new (2). However, since early 2020 this interrelationship has been further exposed and exacerbated by the unprecedented COVID-19 pandemic, which is testing governments, communities, economies, and individuals in ways previously unimagined in their scope and intensity (3). The crisis is exposing underlying inequalities in health and the cost of inaction to address this long-standing social injustice, and the COVID-19 response is even reversing improvements in social and health indicators made in the last two decades (3, 4).[…]
Vaccine
Volume 39, Issue 5 Pages 777-864 (29 January 2021)
https://www.sciencedirect.com/journal/vaccine/vol/39/issue/5
Short communication Full text access
Childhood vaccinations: Hidden impact of COVID-19 on children in Singapore
Youjia Zhong, Hannah Eleanor Clapham, Ramkumar Aishworiya, Ying Xian Chua, … Hui-Lin Chin
Pages 780-785
Vaccine
Volume 39, Issue 5 Pages 777-864 (29 January 2021)
https://www.sciencedirect.com/journal/vaccine/vol/39/issue/5
Review article Open access
Regulatory Harmonization and Streamlining of Clinical Trial Applications globally should lead to faster clinical development and earlier access to life-saving vaccines
Lorenz Scheppler, Norbert De Clercq, Mic McGoldrick, Jacqueline Dias
Pages 790-796
Vaccine
Volume 39, Issue 5 Pages 777-864 (29 January 2021)
https://www.sciencedirect.com/journal/vaccine/vol/39/issue/5
Research article Full text access
Constructing an ethical framework for priority allocation of pandemic vaccines
J Fielding, S.G. Sullivan, F. Beard, K. Macartney, … J. McVernon
Pages 797-804
Vaccine
Volume 39, Issue 5 Pages 777-864 (29 January 2021)
https://www.sciencedirect.com/journal/vaccine/vol/39/issue/5
Research article Abstract only
Timing of COVID-19 vaccine approval and endorsement by public figures
Scott E. Bokemper, Gregory A. Huber, Alan S. Gerber, Erin K. James, Saad B. Omer
Pages 825-829
Vaccine
Volume 39, Issue 5 Pages 777-864 (29 January 2021)
https://www.sciencedirect.com/journal/vaccine/vol/39/issue/5
Research article Open access
Impacts of free vaccination policy and associated factors on influenza vaccination behavior of the elderly in China: A quasi-experimental study
Xuewen Jiang, Xiaopeng Shang, Junfen Lin, Yanrong Zhao, … Yinwei Qiu
Pages 846-852
Vaccines — Open Access Journal
http://www.mdpi.com/journal/vaccines
(Accessed 23 Jan 2021)
Open Access Communication
The Use of Nanobiotechnology in Immunology and Vaccination
by Reza Keikha, Karim Daliri and Ali Jebali
Vaccines 2021, 9(2), 74; https://doi.org/10.3390/vaccines9020074 – 21 Jan 2021
Abstract
Nanotechnology uses the unique properties of nanostructures with a size of 1 to 200 nanometers. Different nanoparticles have shown great promise for the production of new vaccines and drugs. Nanostructures can be used to deliver immunological compounds more effectively than microstructures to target […]
Vaccines — Open Access Journal
http://www.mdpi.com/journal/vaccines
(Accessed 23 Jan 2021)
Open Access Review
A Rapid Systematic Review of Public Responses to Health Messages Encouraging Vaccination against Infectious Diseases in a Pandemic or Epidemic
Vaccines — Open Access Journal
http://www.mdpi.com/journal/vaccines
(Accessed 23 Jan 2021)
Open Access Article
HPV Vaccination Attitudes and Behaviors among General Practitioners in Italy
by Francesco Napolitano, Concetta Paola Pelullo, Giorgia Della Polla and Italo Francesco Angelillo
Vaccines 2021, 9(1), 63; https://doi.org/10.3390/vaccines9010063 – 19 Jan 2021
Abstract
This cross-sectional electronic online or telephone survey assessed the attitudes and behaviors regarding human papillomavirus (HPV) vaccination and the effect of different factors among a nationally representative random sample of 349 general practitioners (GPs) in Italy. A semi-structured interview was performed between September […]
Media/Policy Watch
This watch section is intended to alert readers to substantive news, analysis and opinion from the general media and selected think tanks and similar organizations on vaccines, immunization, global public health and related themes. Media Watch is not intended to be exhaustive, but indicative of themes and issues CVEP is actively tracking. This section will grow from an initial base of newspapers, magazines and blog sources, and is segregated from Journal Watch above which scans the peer-reviewed journal ecology.
We acknowledge the Western/Northern bias in this initial selection of titles and invite suggestions for expanded coverage. We are conservative in our outlook in adding news sources which largely report on primary content we are already covering above. Many electronic media sources have tiered, fee-based subscription models for access. We will provide full-text where content is published without restriction, but most publications require registration and some subscription level.
The Atlantic
http://www.theatlantic.com/magazine/
Accessed 23 Jan 2021
Health
Why Kids Might Be Key to Reaching Herd Immunity
Children rarely get very ill from COVID-19. But there’s another reason to vaccinate them.
Sarah Zhang
January 21, 2021
BBC
http://www.bbc.co.uk/
Accessed 23 Jan 2021
[No new, unique, relevant content]
The Economist
http://www.economist.com/
Accessed 23 Jan 2021
A call for arms
Asian governments are needlessly hampering vaccination drives
Nationalism and geopolitics, among other things, are slowing inoculations
Jan 23rd 2021 edition
Financial Times
https://www.ft.com/
Accessed 23 Jan 2021
Coronavirus treatment
Italy’s Conte threatens to sue vaccine makers over delayed doses
January 23, 2021
Top of Form
Bottom of Form
News in-depth North Korea
Kim Jong Un faces Covid dilemma of isolation or vaccination
International health experts ready to deploy jabs in North Korea — if they are allowed in
January 23, 2021
…North Korea has signalled its interest in participating in the Covax programme, which is seeking to ensure equitable vaccine access around the world, according to people familiar with the matter.
Top of Form
Bottom of Form
Forbes
http://www.forbes.com/
Accessed 23 Jan 2021
10 hours ago
Trump’s Top Health Officials –Fauci, Birx, Redfield – Now Unload Frustrations With Ex-President
Dr. Anthony Fauci said he was ‘blocked’ from going on Maddow, and Dr. Deborah Birx revealed she ‘always’ considered quitting.
By Andrew Solender Forbes Staff
Jan 22, 2021
United Airlines Wants To Make Vaccines Mandatory For Workers
“I think the right thing to do is for United Airlines, and for other companies, to require the vaccines and to make them mandatory,” CEO Scott Kirby said.
By Rachel Sandler Forbes Staff
Jan 22, 2021
Fauci: Trump’s Covid-19 Response ‘Very Likely Did’ Cost Lives
Unlike Trump, Biden plans to “let the science speak,” Fauci said.
By Alison Durkee Forbes Staff
Jan 21, 2021
Fauci Praises World Health Organization’s Pandemic Leadership, Says U.S. Will Rejoin
The decision is a departure from the Trump Administration’s efforts to leave the organization for being too “China-centric.”
By Robert Hart Forbes Staff
Foreign Affairs
http://www.foreignaffairs.com/
Accessed 23 Jan 2021
[No new, unique, relevant content]
Foreign Policy
http://foreignpolicy.com/
Accessed 23 Jan 2021
Argument
India’s Vaccine Diplomacy
The world’s pharmacy is looking to inoculations to build friendly ties around the world—and compete with China.
By Harsh V. Pant, Aarshi Tirkey
| January 22, 2021, 3:39 PM
The Guardian
http://www.guardiannews.com/
Accessed 23 Jan 2021
[No new, unique, relevant content]
New Yorker
http://www.newyorker.com/
Accessed 23 Jan 2021
[No new, unique, relevant content]
New York Times
http://www.nytimes.com/
Accessed 23 Jan 2021
Business
If Poor Countries Go Unvaccinated, a Study Says, Rich Ones Will Pay
Commissioned by the International Chamber of Commerce, the study concludes that equitable distribution of vaccines is in every country’s economic interest, especially those that depend most on trade. It amounts to a rebuke to the popular notion that sharing vaccines with poor countries is merely a form of charity.
By Peter S. Goodman Jan. 23, 2021
Health
In Crises, Vaccines Can Be Stretched, but Not Easily
Shortages of shots for yellow fever, polio and other diseases have led to innovative solutions even in very poor countries.
By Donald G. McNeil Jr. Jan. 22
Health
Pfizer Will Ship Fewer Vaccine Vials to Account for ‘Extra’ Doses
After the surprise discovery of an extra dose in every vial, Pfizer executives successfully lobbied the F.D.A. to change the vaccine’s formal authorization language. The company charges by the dose.
By Noah Weiland, Katie Thomas and Sharon LaFraniere Jan. 22
Health
Biden Inherits a Vaccine Supply Unlikely to Grow Before April
But with 200 million doses pledged for the first quarter of the year, some experts say President Biden’s plan for 100 million shots in 100 days is far too modest.
By Sharon LaFraniere and Noah Weiland
Washington Post
https://www.washingtonpost.com/
Accessed 23 Jan 2021
Elderly begin to drop out of Novavax vaccine trial to get Pfizer and Moderna shots
Christopher Rowland · Business · Jan 19, 2021
Ontario asks Biden for a million vaccines amid shortage
Jan 19, 2021
Think Tanks et al
Brookings
http://www.brookings.edu/
Accessed 23 Jan 2021
[No new relevant content]
Center for Global Development [to 23 Jan 2021]
http://www.cgdev.org/page/press-center
[No new relevant content]
Chatham House [to 23 Jan 2021]
https://www.chathamhouse.org/
Accessed 23 Jan 2021
[No new relevant content]
CSIS
https://www.csis.org/
Accessed 23 Jan 2021
Upcoming Event
Online Event: The State of Immunization Under Covid-19
January 29, 2021
Upcoming Event
Online Event: Trusting a Covid-19 Vaccine: The Role of the Media and Misinformation
January 27, 2021
Council on Foreign Relations
http://www.cfr.org/
Accessed 23 Jan 2021
January 22, 2021
Pharmaceuticals and Vaccines
What to Know About the Global COVID-19 Vaccine Rollout So Far
Several countries stand out for their success in delivering coronavirus vaccinations, while most of the world is struggling to figure out how to get immunizations into more arms.
In Brief by Claire Felter
Kaiser Family Foundation
https://www.kff.org/search/?post_type=press-release
Accessed 23 Jan 2021
January 22, 2021 News Release
New Resources Track State Vaccinations by Race/Ethnicity and Examine Demographics of Health Workers
A new Policy Watch, Early State Vaccination Data Raise Warning Flags for Racial Equity, explores the latest state-reported data on vaccination by race/ethnicity available on KFF’s COVID-19 state data and policy tracker. As of January 19, 2021, 17 states were reporting some vaccination data by race/ethnicity, including 16 states reporting…
January 22, 2021 News Release
Nearly 6 in 10 Older Americans Don’t Know When or Where They Can Get a COVID-19 Vaccine; Black and Hispanic Adults among the Groups Least Likely to Have Enough Information
Despite Optimism about COVID-19 Vaccines in the Future, Half Say They are Frustrated with the Current Situation and Nearly a Quarter are Angry While older Americans are a high-priority group for getting a COVID-19 vaccine, the latest KFF COVID-19 Vaccine Monitor report finds that, among those who have not yet been vaccinated,…
World Economic Forum [to 23 Jan 2021]
https://agenda.weforum.org/news/
Media
[No new relevant content]
Vaccines and Global Health: The Week in Review is a weekly digest summarizing news, events, announcements, peer-reviewed articles and research in the global vaccine ethics and policy space. Content is aggregated from key governmental, NGO, international organization and industry sources, key peer-reviewed journals, and other media channels. This summary proceeds from the broad base of themes and issues monitored by the Center for Vaccine Ethics & Policy in its work: it is not intended to be exhaustive in its coverage. You are viewing the blog version of our weekly digest, typically comprised of between 30 and 40 posts below all dated with the current issue date
.– Request an Email Summary: Vaccines and Global Health : The Week in Review is published as a single email summary, scheduled for release each Saturday evening before midnight (EDT in the U.S.). If you would like to receive the email version, please send your request to david.r.curry@centerforvaccineethicsandpolicy.org.
– pdf version: A pdf of the current issue is available here:
– blog edition: comprised of the approx. 35+ entries posted below.
– Twitter: Readers can also follow developments on twitter: @vaxethicspolicy.
.
– Links: We endeavor to test each link as we incorporate it into any post, but recognize that some links may become “stale” as publications and websites reorganize content over time. We apologize in advance for any links that may not be operative. We believe the contextual information in a given post should allow retrieval, but please contact us as above for assistance if necessary.
Support this knowledge-sharing service: Your financial support helps us cover our costs and to address a current shortfall in our annual operating budget. Click here to donate and thank you in advance for your contribution.
.
David R. Curry, MS
Executive Director
Center for Vaccine Ethics and Policy
Milestones :: Perspectives :: Research
WHO – 148th session of the Executive Board EB148
18-26 January 2021
Main Documents [Selected; Editor’s text bolding]
EB148/1
Provisional agenda
EB148/1(annotated)
Provisional agenda (annotated)
EB148/2
Opening of the session and adoption of the agenda
Special procedures
EB148/4
Report of the regional committees to the Executive Board
EB148/6
Global action on patient safety
EB148/7
Political declaration of the third high-level meeting of the General Assembly on the prevention and control of non-communicable diseases
EB148/7 Add.1
Political declaration of the third high-level meeting of the General Assembly on the prevention and control of non-communicable diseases
Mid-point evaluation of the implementation of the WHO global action plan for the prevention and control of noncommunicable diseases 2013–2020
Executive summary
EB148/7 Add.2
Political declaration of the third high-level meeting of the General Assembly on the prevention and control of non-communicable diseases
Final evaluation of the global coordination mechanism on the prevention and control of noncommunicable diseases
Executive summary
EB148/8
Oral health
Achieving better oral health as part of the universal health coverage and noncommunicable disease agendas towards 2030
EB148/9
Expanding access to effective treatments for cancer and rare and orphan diseases, including medicines, vaccines, medical devices, diagnostics, assistive products, cell- and gene-based therapies and other health technologies; and improving the transparency of markets for medicines, vaccines, and other health products
EB148/10
Global strategy and plan of action on public health, innovation and intellectual property
EB148/11
Antimicrobial resistance
EB148/12
Substandard and falsified medical products
EB148/13
Standardization of medical devices nomenclature
EB148/14
Immunization Agenda 2030
EB148/15
Integrated people-centred eye care, including preventable vision impairment and blindness
EB148/16
COVID-19 response
EB148/17
Public health emergencies: preparedness and response
WHO’s work in health emergencies
EB148/18
WHO’s work in health emergencies
Strengthening WHO’s global emergency preparedness and response
EB148/19
Strengthening preparedness for health emergencies: implementation of the International Health Regulations (2005)
Interim progress report of the Review Committee on the Functioning of the International Health Regulations (2005) during the COVID-19 Response
EB148/20
Mental health preparedness and response for the COVID-19 pandemic
EB148/21
The public health implications of implementation of the Nagoya Protocol
EB148/22
Poliomyelitis
Poliomyelitis eradication
EB148/23
Poliomyelitis
Polio transition planning and polio post-certification
EB148/24
Social determinants of health
Milestones :: Perspectives :: Research
OCV
UNICEF, WHO, IFRC and MSF announce the establishment of a global Ebola vaccine stockpile
NEW YORK/ GENEVA, 12 JANUARY 2021: The four leading international health and humanitarian organizations announced today the establishment of a global Ebola vaccine stockpile to ensure outbreak response.
The effort to establish the stockpile was led by the International Coordinating Group (ICG) on Vaccine Provision, which includes the World Health Organization (WHO), UNICEF, the International Federation of Red Cross and Red Crescent Societies (IFRC), and Médecins Sans Frontières (MSF), with financial support from Gavi, the Vaccine Alliance. The stockpile will allow countries, with the support of humanitarian organizations, to contain future Ebola epidemics by ensuring timely access to vaccines for populations at risk during outbreaks.
The injectable single-dose Ebola vaccine (rVSV∆G-ZEBOV-GP, live) is manufactured by Merck, Sharp & Dohme (MSD) Corp. and developed with financial support from the US government. The European Medicines Agency licensed the Ebola vaccine in November 2019, and the vaccine is now prequalified by WHO, and licensed by the US Food and Drug Administration as well as in eight African countries.
Before achieving licensure, the vaccine was administered to more than 350,000 people in Guinea and in the 2018-2020 Ebola outbreaks in the Democratic Republic of the Congo under a protocol for “compassionate use”.
The vaccine, which is recommended by the Strategic Advisory Group of Experts (SAGE) on Immunization for use in Ebola outbreaks as part of a broader set of Ebola outbreak response tools, protects against the Zaire ebolavirus species which is most commonly known to cause outbreaks.
“The COVID-19 pandemic is reminding us of the incredible power of vaccines to save lives from deadly viruses,” said Dr Tedros Adhanom Ghebreyesus, WHO Director-General. “Ebola vaccines have made one of the most feared diseases on earth preventable. This new stockpile is an excellent example of solidarity, science and cooperation between international organizations and the private sector to save lives.”
UNICEF manages the stockpile on behalf of the ICG which, as with stockpiles of cholera, meningitis and yellow fever vaccines, will be the decision-making body for its allocation and release…
Milestones :: Perspectives :: Research
Coronavirus [COVID-19] – WHO
Public Health Emergency of International Concern (PHEIC)
Weekly Epidemiological and Operational updates
Last update: 16 January 2021
Confirmed cases :: 92 506 811 [week ago: 87 589 206] [two weeks ago: 82 356 727
Confirmed deaths :: 2 001 773 [week ago: 1 906 606] [two weeks ago: 1 815 433]
Countries, areas or territories with cases :: 223
Weekly epidemiological update – 12 January 2021
Overview
Following two weeks of low reporting, likely due to the year-end and holiday period, the overall upward trend seen prior to this period is continuing, with just under 5 million new cases reported last week globally. This brings the cumulative numbers to over 88 million reported cases and over 1.9 million deaths globally since the start of the pandemic.
In this issue, we summarize the current epidemiological situation regarding SARS-CoV-2 variants of concern.
::::::
Statement on the sixth meeting of the International Health Regulations (2005) Emergency Committee regarding the coronavirus disease (COVID-19) pandemic
15 January 2021 Statement
The sixth meeting of the Emergency Committee convened by the WHO Director-General under the International Health Regulations (2005) (IHR) regarding the coronavirus disease (COVID-19) took place on Thursday, 14 January 2021 from 12:15 to 16:45 Geneva time (CEST)….
…The WHO Director of the Immunization, Vaccines and Biologicals Department presented the current status of the COVID-19 vaccine landscape and introduction. The Chair of the Strategic Advisory Group of Experts on Immunization (SAGE) noted available guidance including WHO SAGE Roadmap for Prioritizing Uses of COVID-19 Vaccines in the Context of Limited Supply and the Interim Recommendations for Use of the Pfizer-BioNTech COVID-19 Vaccine (BNT162b2) under Emergency Use Listing.
The Director of Air Transport Bureau of the International Civil Aviation Organization (ICAO) shared their COVID-19 activities related to testing and vaccination, including the Manual on Testing and Cross Border Risk Management Measures (Doc 10152) which provides countries with risk management strategies for international travel. The WHO Unit Head of the IHR Secretariat provided an overview of the legal provisions as well as the scientific, ethical and technological considerations for vaccination certificates related to international travel.
The Committee recognized the challenges posed by some manufacturers’ delayed submission of vaccine data to WHO. These data delays impact WHO’s ability to provide emergency use listing which ultimately affect equitable vaccine access. The Committee strongly encourages manufacturers to provide data to WHO as rapidly as possible.
The Committee unanimously agreed that the COVID-19 pandemic still constitutes an extraordinary event, a public health risk to other States through international spread, and continues to require a coordinated international response. As such, the Committee concurred that the COVID-19 pandemic remains a public health emergency of international concern (PHEIC) and offered advice to the Director-General.
The Committee recognized WHO’s and States Parties’ progress in implementing the previous Temporary Recommendations from the 5th meeting of the Emergency Committee. The Committee noted that these recommendations remain relevant and had acquired additional urgency given the evolution of the pandemic and the continued need for a coordinated global response. The Committee advised on extending the previous Temporary Recommendations and provided additional advice to the Director-General.
The Director-General determined that the COVID-19 pandemic continues to constitute a PHEIC. He accepted the advice of the Committee to WHO and issued the Committee’s advice to States Parties as Temporary Recommendations under the IHR.
The Emergency Committee will be reconvened within three months, at the discretion of the Director-General. The Director-General thanked the Committee for its work…
Specific Recommendations made by the Emergency Committee to the WHO Secretariat and Additional Temporary Recommendations to State Parties are available here.
::::::
IHR Emergency Committee on COVID-19 advises on variants, vaccines
15 January 2021 News release
The COVID-19 pandemic continues to constitute a Public Health Emergency of International Concern (PHEIC), according to the WHO Emergency Committee (EC) on COVID-19.
The EC met virtually yesterday (14 January) at the request of WHO Director-General Dr Tedros Adhanom Ghebreyesus to review the emerging variants of SARS-CoV-2, the virus that causes COVID-19, and to consider the potential use of vaccination and testing certificates for international travel.
On variants, the EC called for a global expansion of genomic sequencing and sharing of data, along with greater scientific collaboration to address critical unknowns.
The committee urged WHO to develop a standardized system for naming new variants that avoids geographical markers, an area WHO has already begun work on.
On vaccines, the committee underlined the need for equitable access through the COVAX Facility as well as technology transfer to increase global production capacities.
The committee strongly encouraged vaccine manufacturers to rapidly provide safety and efficacy data to WHO for emergency use listing. The lack of such data is a barrier to ensuring the timely and equitable supply of vaccines at the global level…
::::::
16 January 2021 News release
Scientists tackle vaccine safety, efficacy and access at global R&D forum
More than 2,800 scientists from 130 countries gathered on Friday (January 15) in a virtual forum hosted by the World Health Organization (WHO) to identify knowledge gaps and set research priorities for vaccines against SARS-CoV-2, the virus that causes COVID-19.
They discussed the safety and efficacy of existing vaccines and new candidates, ways to optimize limited supply, and the need for additional safety studies.
“The development and approval of several safe and effective vaccines less than a year after this virus was isolated and sequenced is an astounding scientific accomplishment,” said Dr Tedros Adhanom Ghebreyesus, WHO Director-General, in his opening remarks. “The approval of the first few vaccines does not mean the job is done. Far from it. More vaccines are in the pipeline, which must be evaluated to ensure we have enough doses to vaccinate everyone.”
More than 30 million vaccine doses have already been administered in 47 mostly high-income countries. But the global vaccine rollout has exposed glaring inequalities in access to this life-saving tool…
…The meeting concluded with agreement to establish a WHO-hosted platform for global sharing and coordination of emerging vaccine research information on efficacy and safety. The forum would enable scientists to share and discuss unpublished and published data and research protocols to further our collective understanding of SARS-CoV-2 vaccines.
“The WHO will regularly convene experts from around the world, promote collaborative research, provide standard protocols and develop a platform for sharing the latest knowledge in the field,” said Dr Soumya Swaminathan, WHO Chief Scientist.
Milestones :: Perspectives :: Research
COVID: WHO SAGE; IVB
Extraordinary meeting of the Strategic Advisory Group of Experts on Immunization (SAGE)
21 January 2021 10:00 – 17:00 CET
This extraordinary virtual meeting for the Strategic Advisory Group of Experts on Immunization (SAGE) will tentatively be scheduled on Thursday 21 January 2021 to propose recommendations to WHO on the use of COVID-19 vaccine(s) [Moderna mRNA-1273]
Draft Agenda
::::::
Background document on mRNA vaccine BNT162b2 (Pfizer-BioNTech) against COVID-19
Background document to the WHO Interim recommendations for use of the Pfizer–BioNTech COVID-19 vaccine, BNT162b2, under Emergency Use Listing
14 January 2021
Technical document: PDF – https://apps.who.int/iris/rest/bitstreams/1327316/retrieve
Interim recommendations for use of the Pfizer–BioNTech COVID-19 vaccine, BNT162b2, under Emergency Use Listing
WHO Team: WHO Headquarters (HQ)
Reference numbers: WHO Reference Number: WHO/2019-nCoV/vaccines/SAGE_recommendation/BNT162b2/2021.1
Copyright CC BY-NC-SA 3.0 IGO
Full text PDF: https://apps.who.int/iris/rest/bitstreams/1326072/retrieve [7 pages]
Milestones :: Perspectives :: Research
UNICEF COVID-19 Vaccine Market Dashboard :: Agreements Table Accessed 16 Jan 2021
An overview of information collected from publicly announced bilateral and multilateral supply agreement [Agreements view from 2021-1-05 to date]
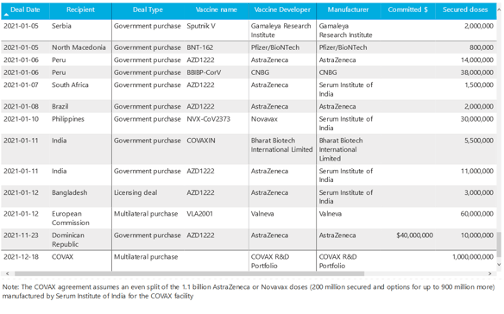
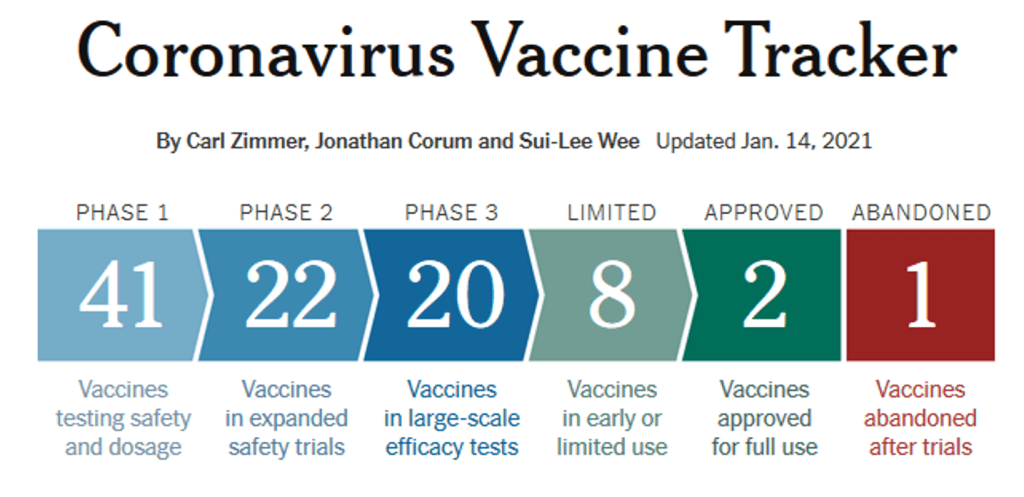
Milestones :: Perspectives :: Research
New York Times :: Coronavirus Vaccine Tracker
14 Jan 2021
New additions and recent updates
Jan. 14
The Israel Institute for Biological Research moves to Phase 2.
Jan. 13
Brazil announces Sinovac’s vaccine has an efficacy of just over 50 percent.
Jan. 12
California-based Arcturus moves to Phase 2.
Jan. 12
Canada’s VIDO enters Phase 1/.

Milestones :: Perspectives :: Research
Innovative Biopharmaceutical Industry Comment on COVID-19 Vaccines Dosing Strategies and Recommend Following the Science
This statement on COVID-19 vaccine dosing strategies was issued by:
:: the Pharmaceutical Research and Manufacturers of America (PhRMA),
:: the International Federation of Pharmaceutical Manufacturers & Associations (IFPMA),
:: International Council of Biotech Associations (ICBA),
:: the Biotechnology Innovation Organization (BIO),
:: the European Federation of Pharmaceutical Industries and Associations (EFPIA) and
:: Vaccines Europe.
January 13, 2021
“With the first COVID-19 vaccines authorized by regulatory authorities and administered to the public, the biopharmaceutical industry, including leading vaccine makers, recognize that a critical new phase of the COVID-19 pandemic has begun. Collaboration among the global community has resulted in safe and efficacious vaccines being authorized by stringent regulatory agencies, which are already being manufactured and distributed. Additional vaccines are expected to follow.
“The biopharmaceutical industry acknowledges the considerable challenges governments are facing to urgently address the enormous strain the pandemic is placing on healthcare systems, societies and economies. In light of the urgent need to reach as many people as possible with COVID-19 vaccines, there are emerging discussions regarding dosing strategies that may not be supported by the authorized labeling or published clinical data.
“The biopharmaceutical industry commits to work in partnership with regulatory agencies and recommending bodies to gather further clinical data on several ongoing scientific questions with regard to COVID-19 vaccines. The innovative biopharmaceutical industry believes that vaccine deployment strategies should be based on the outcome of these continuing clinical studies and the evolving knowledge. Therefore, the biopharmaceutical industry supports adhering to the dosing that has been assessed in clinical trials and urges that any changes from the tested and approved vaccine dosing and vaccination schedules for COVID-19 vaccines should follow the science and be based on a transparent deliberation of the available data.
“It is vital to preserve, build and sustain public confidence in COVID-19 vaccination by continuing to make and communicate policy decisions based on robust scientific evidence. Only then can we bring this pandemic to an end.
“The biopharmaceutical industry will continue to develop and test vaccine candidates for COVID-19 through a sound, scientific and deliberative process. Vaccine manufacturers have pledged to only submit vaccine candidates for approval or emergency use authorization after demonstrating safety and efficacy in clinical trials that are designed and conducted to meet the requirements of regulatory authorities.1 Our collective membership remains committed to continuing to share our findings with regulatory authorities, public health experts and academics to assist with evidence-based decision-making.”
Milestones :: Perspectives :: Research
Vaccination Credential Initiative (VCI)
Broad Coalition of Health and Technology Industry Leaders Announce Vaccination Credential Initiative to Accelerate Digital Access to COVID-19 Vaccination Records
:: The Vaccination Credential Initiative (VCI) is working to enable individuals vaccinated for COVID-19 to access their vaccination records in a secure, verifiable and privacy-preserving way.
:: Coalition partners include CARIN Alliance, Cerner, Change Healthcare, The Commons Project Foundation, Epic, Evernorth, Mayo Clinic, Microsoft, MITRE, Oracle, Safe Health, Salesforce.
:: The coalition is developing a standard model for organizations administering COVID-19 vaccines to make credentials available in an accessible, interoperable, digital format.
:: Trustworthy, traceable, verifiable, and universally recognized digital record of vaccination status is urgently needed worldwide to safely enable people to return to work, school, events, and travel.
January 14, 2021 (NEW YORK): A broad coalition of health and technology leaders today announced the creation of the Vaccination Credential Initiative (VCI), committed to empowering individuals with digital access to their vaccination records based on open, interoperable standards.
The current vaccination record system does not readily support convenient access, control and sharing of verifiable vaccination records. VCI coalition members are working to enable digital access to vaccination records using the open, interoperable SMART Health Cards specification, based on W3C Verifiable Credential and HL7 FHIR standards.
VCI’s vision is to empower individuals to obtain an encrypted digital copy of their immunization credentials to store in a digital wallet of their choice. Those without smartphones could receive paper printed with QR codes containing W3C verifiable credentials.
“The goal of the Vaccination Credential Initiative is to empower individuals with digital access to their vaccination records so they can use tools like CommonPass to safely return to travel, work, school, and life, while protecting their data privacy,” said Paul Meyer, CEO of The Commons Project Foundation. “Open standards and interoperability are at the heart of VCI’s efforts and we look forward to supporting the World Health Organization and other global stakeholders in implementing and scaling open global standards for health data interoperability.”
“As we explore the many use cases for the vaccination credential, we are working to ensure that underserved populations have access to this verification,” said Dr. Brian Anderson, chief digital health physician at MITRE. “Just as COVID-19 does not discriminate based on socio-economic status, we must ensure that convenient access to records crosses the digital divide. MITRE is an independent advisor and trusted source for managing third-party data and proud to be joining with The Commons Project and other coalition members to deliver an open-source credential.”…
“Salesforce is proud to join the Vaccination Credential Initiative to help organizations easily and safely customize all aspects of the vaccination management lifecycle and integrate closely with other coalition members’ offerings, which will help us all get back to public life,” said Bill Patterson, executive vice president and general manager, CRM Applications at Salesforce. “With a single platform to help deliver safe and continuous operations and deepen trust with customers and employees, this coalition will be crucial to support public health and wellbeing.”…
“We are kicking off the most significant vaccination effort in the history of the United States. Now more than ever, individuals need access to their own vaccination and health information in a portable format to begin to move about the country safely and comfortably,” said Ryan Howells, principal, Leavitt Partners and program manager of the CARIN Alliance. “The CARIN Alliance is supportive of MITRE’s effort to provide individuals with access to their vaccination information in a secure and trusted way and looks forward to advising the VCI initiative on ways to leverage the CARIN code of conduct and other best practices to facilitate consumer-directed exchange that we have developed consensus on over the last few years.”
Milestones :: Perspectives :: Research
COVID Vaccines – FDA
No new major regulatory announcements on COVID vaccines identified
Milestones :: Perspectives :: Research
COVID Vaccines – EMA
Meeting highlights from the Pharmacovigilance Risk Assessment Committee (PRAC) 11-14 January 2021
News 15/01/2021
First monthly summary safety report for COVID-19 vaccine Comirnaty
Starting this month, EMA’s safety committee (PRAC) will evaluate summary safety reports submitted monthly by marketing authorisations holders of COVID-19 vaccines. The first such report will be for Comirnaty.
The company is expected to submit their monthly summary safety report in mid-January. The PRAC will evaluate and discuss it during its PRAC plenary virtual meeting at the end of January.
The outcome of the PRAC’s assessment will be communicated on EMA’s website shortly after.
During the pandemic, marketing authorisation holders for COVID-19 vaccines are expected to submit monthly summary safety reports, in line with the risk management plan and as described in the safety monitoring plan for COVID-19 vaccines prepared by EMA and the national competent authorities of the EU Member States. The plan outlines how relevant new information emerging after the authorisation and roll-out of COVID-19 vaccines will be collected and promptly reviewed.
The monthly summary safety report will include, among others, information on reported suspected adverse reactions, including adverse events of special interest (AESIs).
The submission of such reports complements the submission of periodic safety update reports (PSURs)…
News: EMA receives application for conditional marketing authorisation of COVID-19 Vaccine AstraZeneca
Last updated: 12/01/2021
Milestones :: Perspectives :: Research
U.K.
Medicines and Healthcare products Regulatory Agency (MHRA)
https://www.gov.uk/coronavirus
Announcements
More than a third of over 80s vaccinated against COVID-19
Published 14 January 2021
U.K. Joint Committee on Vaccination and Immunisation (JCVI)
https://www.gov.uk/search/all?keywords=JCVI
No new announcement activity identified
Milestones :: Perspectives :: Research
COVID Vaccines: Company Announcements on Development/Regulatory Actions/Procurement/Deployment
Dr. Reddy’s Receives Approval to Conduct Phase 3 Clinical Trial for Sputnik V Vaccine in India
January 15, 2021
Dr. Reddy’s Laboratories Ltd (BSE: 500124, NSE: DRREDDY, NYSE: RDY, NSEIFSC: DRREDDY) announced today that it has received approval from the Drugs Control General of India (DCGI) to conduct phase 3 clinical trial for the Sputnik V vaccine in India. The phase 3 study of Sputnik V will be conducted on 1500 subjects as part of the randomized, double-blind, parallel-group, placebo-controlled study in India//.
Bharat Biotech Signs Agreement with Precisa Medicamentos For Supply of ‘COVAXINTM’ to Brazil
Hyderabad, January 12, 2020:Bharat Biotech announced that it has signed an agreement with Precisa Medicamentos for the supplies of COVAXIN™ to Brazil…
In principle, it is understood between both parties that supplies of COVAXIN™ to be prioritized for the public market, through a direct procurement by the Govt. of Brazil. Supplies to the private market would be based upon receipt of market authorization from ANVISA, the Brazilian regulatory authority…
Swissmedic Authorizes COVID-19 Vaccine Moderna for Use in Switzerland
Swiss Federal Government has secured 7.5 million doses and first deliveries expected to begin in January
January 12, 2021
CAMBRIDGE, Mass.–(BUSINESS WIRE)–Moderna, Inc. (Nasdaq: MRNA), a biotechnology company pioneering messenger RNA (mRNA) therapeutics and vaccines, today announced that Swissmedic, the Swiss Agency for Therapeutic Products, has authorized the COVID-19 Moderna Vaccine in Switzerland…
Sputnik V meets the primary endpoint of safety in the Phase 2 Clinical Trial in India
Dr. Reddy’s submitted the phase 2 safety data for DCGI’s approval to continue phase 3 clinical trials
January 11, 2021
Baxter Biopharma Solutions Announces Sterile Manufacturing Agreement for Novavax COVID-19 Vaccine
:: Agreement will help increase commercial production and promote access in United Kingdom and European markets
:: Manufacturing to take place at Baxter’s state-of-the-art Halle/Westfalen, Germany facility
January 11, 2021
Milestones :: Perspectives :: Research
COVID Vaccines Development/Procurement/Distribution/Policy – Russia, China
Russia: Sputnik V – “the first registered COVID-19 vaccine” [to 16 Jan 2021]
https://sputnikvaccine.com/newsroom/pressreleases/
Press Releases
Sputnik V vaccine approved in Paraguay
Press release, 15.01.2021
… The vaccine was registered under the emergency use authorization procedure without additional clinical trials in the country. Sputnik V had been registered under the same procedure earlier in Algeria, Argentina, Bolivia, Serbia, Palestine and Venezuela.
Supplies of the vaccine will be facilitated by RDIF’s international partners in India, China, South Korea and other countries…
RDIF and União Química to supply 10 million doses of Sputnik V vaccine to Brazil
Press release, 13.01.2021
The Russian Direct Investment Fund (RDIF, Russia’s sovereign wealth fund) and one of the leading pharmaceutical companies of Brazil União Química have agreed to supply to the country 10 million doses of the world’s first registered vaccine against coronavirus Sputnik V in the first quarter of 2021 with deliveries beginning in January…
The Russian Direct Investment Fund (RDIF, Russia’s sovereign wealth fund) and one of the leading pharmaceutical companies of Brazil União Química have agreed to supply to the country 10 million doses of the world’s first registered vaccine against coronavirus Sputnik V in the first quarter of 2021 with deliveries beginning in January…
Algeria has become the first country in Africa to register Sputnik V vaccine
Press release, 10.01.2021
The Russian Direct Investment Fund (RDIF, Russia’s sovereign wealth fund) announces the registration of the Russian Sputnik V vaccine against coronavirus by the National Agency of Pharmaceutical Products of People’s Democratic Republic of Algeria.
The vaccine was registered under the emergency use authorization procedure. Sputnik V has been registered under the same procedure earlier in Argentina, Bolivia and Serbia.
Supplies of the vaccine to Algeria will be facilitated by international partners of RDIF in India, China, South Korea and other countries…
::::::
China: COVID-19 Vaccines – Announcement/Regulatory Actions/Deployment
National Health Commission of the People’s Republic of China [to 16 Jan 2021]
http://en.nhc.gov.cn/
News
China to expand COVID-19 vaccination to include people aged over 60
2021-01-14
COVID-19 vaccine safe, effective, official says
2021-01-11
More than 9 million doses of COVID-19 vaccines have been administered across China, and the safety and efficacy of the vaccines have been proved, a top health official said.
With production increasing, the number of vaccine doses will be extended to cover the whole Chinese population free of charge as a crucial weapon for epidemic prevention and control, Vice-Health Minister Zeng Yixin said over the weekend…
China’s COVID-19 vaccine found capable of neutralizing UK strain
2021-01-11
China’s COVID-19 vaccine is found capable of neutralizing the new strain of the novel coronavirus that was reported to be behind the rise in transmission of the disease in parts of the United Kingdom, senior health official said on Jan 9.
Zeng Yixin, vice-minister of the National Health Commission, said China’s scientific community is paying close attention to the new variant and its effect on current vaccines as reports indicated that the new strain had arrived in China via imported cases.
Scientists from the Institute of Laboratory Animal Sciences of the Chinese Academy of Medical Sciences and the Sun Yat-sen University in Guangdong province are already working on the issue, Zeng said during a news briefing held by the State Council Information Office.
After examining the new viral strain and strains that existed in China from January to June last year, researchers found that the animal and human antibodies induced by China’s COVID-19 vaccine can neutralize the new variant, Zeng said, adding they have submitted their findings to scientific journals and is awaiting publication…
National Health Commission of the People’s Republic of China [to 16 Jan 2021]
http://en.nhc.gov.cn/
WHO team working with Chinese vaccine producers ahead of potential emergency use: WHO director-general
2021-01-13
GENEVA — The World Health Organization (WHO) Director-General Tedros Adhanom Ghebreyesus has said that a WHO team in China is working with producers of the Sinovac and Sinopharm vaccines for potential emergency use listings.
“I’m pleased that a WHO team is in China currently working with producers of the Sinovac and Sinopharm vaccines to assess compliance with international quality manufacturing practices ahead of potential emergency use listing by WHO,” Tedros said at a recent media briefing.
He noted that WHO continues to ask vaccine manufacturers from around the world to move swiftly to provide necessary data that will allow the organization to consider them for emergency use listings.
“While we are hopeful about the safe and effective vaccines that are being rolled out, we want to see this sped up and vaccines allocated equitably in the coming weeks,” Tedros said.
WHO & Regional Offices [to 16 Jan 2021]
13 January 2021 Departmental news
World Health Assembly endorses the 1st ever resolution on meningitis prevention and control
Defeating meningitis by 2030
At the 73rd Session of the World Health Assembly, Member States overwhelmingly endorsed a resolution calling for urgent action on meningitis prevention and control through the implementation of a bold, comprehensive global roadmap to defeat meningitis by 2030. Developed under WHO leadership, through extensive and broad consultation, this global roadmap paves the way for the implementation of multidisciplinary, integrated interventions to achieve:
:: long-term integrated meningitis prevention and control for an accelerated and durable reduction in cases and deaths;
:: shifting from epidemic preparedness and response to prevention and elimination of epidemics;
:: recognition of long-term sequelae from meningitis and concerted action to reduce disability and provide support to people affected and their families…
::::::
Weekly Epidemiological Record, 2021, vol. 96, 01-02 [full issue]
(2021-01-15)
:: Summary of the 31st meeting of the International Task Force for Disease Eradication, 20–21 October 2020
:: Monthly report on dracunculiasis cases, January-October 2020
::::::
WHO Regional Offices
Selected Press Releases, Announcements
WHO African Region AFRO
No new digest content identified
WHO Region of the Americas PAHO
No new digest content identified
WHO South-East Asia Region SEARO
:: 13 January 2021 News release Marking a decade since last polio case: WHO SEAR countries gear up for massive vaccination campaign – this time for COVID-19 virus
WHO European Region EURO
:: A tipping-point in the course of the pandemic 14-01-2021
:: Uzbekistan is making healthy diets a cornerstone of national policy with the help of WHO 12-01-2021
WHO Eastern Mediterranean Region EMRO
No new digest content identified
WHO Western Pacific Region
No new digest content identified
Coronavirus Disease 2019 (COVID-19)– CDC
Selected Resources
:: COVID-19 Vaccine Communication Toolkit for Community-Based Organizations: Getting Started Friday, January 15, 2021
:: CDC Strategy for Global Response to COVID-19 (2020-2023) Friday, January 15, 2021
:: Requirement for Proof of Negative COVID-19 Test or Recovery from COVID-19 for All Air Passengers Arriving in the United States Friday, January 15, 2021
:: Toolkit for Childcare Programs Friday, January 15, 2021
:: COVID-19 Vaccination Wednesday, January 13, 2021
:: Operational Considerations for Immunization Services during COVID-19 in Non-US Settings Focusing on Low-Middle Income Countries Tuesday, January 12, 2021
:: Markets: Operational considerations for COVID-19 mitigation measures in low resource settings Tuesday, January 12, 2021
Africa CDC [to 16 Jan 2021]
http://www.africacdc.org/
News
No new digest content identified.
China CDC
http://www.chinacdc.cn/en/
No new digest content identified.
National Health Commission of the People’s Republic of China [to 16 Jan 2021]
http://en.nhc.gov.cn/
News
WHO team for virus origin-tracing arrives in Wuhan
2021-01-15
An international expert team from the World Health Organization arrived in Wuhan, Hubei province, on Jan 14 to work with its Chinese counterparts on origin-tracing and scientific research of the novel coronavirus.
The team landed at Wuhan Tianhe International Airport on Thursday morning, where they received throat swab and serum antibody tests for the coronavirus before going into a 14-day quarantine, according to China Global Television Network.
Foreign Ministry spokesman Zhao Lijian said at a regular news briefing in Beijing on Thursday that, during their quarantine, the WHO experts will have exchanges via video link with Chinese scientists and medical experts.
All of the team members had multiple tests for COVID-19 in their home countries before traveling.
The WHO confirmed the arrival of the international team of 13 scientists in Wuhan in a post on social media, which also said that another two scientists are still in Singapore to be retested after they tested positive for antibodies associated with COVID-19…
China to expand COVID-19 vaccination to include people aged over 60
2021-01-14
COVID-19 vaccine safe, effective, official says
2021-01-11
[See COVID – CHINA above for detail]
China’s COVID-19 vaccine found capable of neutralizing UK strain
2021-01-11
[See COVID – CHINA above for detail]
National Medical Products Administration [to 16 Jan 2021]
http://english.nmpa.gov.cn/news.html
News
Chinese mainland reports 135 new locally transmitted COVID-19 cases
2021-01-15
The Chinese mainland on Jan 14 reported 144 newly confirmed COVID-19 cases, of whom 135 were locally transmitted and the rest arrived from outside the mainland, the National Health Commission said on Jan 15.
Public likely to receive vaccines in February
2021-01-14
Vaccinations for the general public against COVID-19 are expected to start around Spring Festival, which begins on Feb 12, China’s health authorities said.
WHO team working with Chinese vaccine producers ahead of potential emergency use: WHO director-general
2021-01-13
[See COVID – CHINA above for detail]
Organization Announcements
Paul G. Allen Frontiers Group [to 16 Jan 2021]
https://alleninstitute.org/what-we-do/frontiers-group/news-press/
News
No new digest content identified.
BARDA – U.S. Department of HHS [to 16 Jan 2021]
https://www.phe.gov/about/barda/Pages/default.aspx
BARDA News
January 12, 2021: HHS, DOD purchase additional doses of Regeneron’s antibody therapeutic to treat patients with mild to moderate COVID-19
BMGF – Gates Foundation [to 16 Jan 2021]
http://www.gatesfoundation.org/Media-Center/Press-Releases
Press Releases and Statements
No new digest content identified.
Bill & Melinda Gates Medical Research Institute [to 16 Jan 2021]
https://www.gatesmri.org/
The Bill & Melinda Gates Medical Research Institute is a non-profit biotech organization. Our mission is to develop products to fight malaria, tuberculosis, and diarrheal diseases—three major causes of mortality, poverty, and inequality in developing countries. The world has unprecedented scientific tools at its disposal; now is the time to use them to save the lives of the world’s poorest people
No new digest content identified.
CARB-X [to 16 Jan 2021]
https://carb-x.org/
News
No new digest content identified.
Center for Vaccine Ethics and Policy – GE2P2 Global Foundation [to 16 Jan 2021]
https://centerforvaccineethicsandpolicy.net/
News/Analysis/Statements
No new digest content identified.
CEPI – Coalition for Epidemic Preparedness Innovations [to 16 Jan 2021]
http://cepi.net/
Latest News
The importance of blood markers in assessing vaccine efficacy
13 Jan 2021
By Mario Christodoulou
EDCTP [to 16 Jan 2021]
http://www.edctp.org/
The European & Developing Countries Clinical Trials Partnership (EDCTP) aims to accelerate the development of new or improved drugs, vaccines, microbicides and diagnostics against HIV/AIDS, tuberculosis and malaria as well as other poverty-related and neglected infectious diseases in sub-Saharan Africa, with a focus on phase II and III clinical trials
13 January 2021
Public consultation: Global Roadmap for research and development for TB vaccines
EDCTP has commissioned the Amsterdam Institute for Global Health and Development (AIGHD) to develop a Global Roadmap for research and development for tuberculosis (TB) vaccines. The Roadmap intents to provide global stakeholders such as researchers, funders, industry, regulatory and policy decision makers with key actionable priorities that could help guide their actions. It lists both the short-term objectives as well as the long-term strategic objectives for global TB vaccine development, and focuses on developing and delivering affordable and effective vaccines for use in low- and middle-income countries where the vast majority of people affected by TB are concentrated.
AIGHD and EDCTP invite researchers and stakeholders, as well as everyone who has been involved in the roadmap development process to respond to a public consultation and express their views on aspects of the draft Roadmap before a final version is published.
This is part of the process for developing this Roadmap, which consists of several steps:
:: Desk review and stakeholder inventory
:: In-depth interviews with selected stakeholders
:: A consensus workshop and various rounds of stakeholder consultation on the subsequent draft versions of the Roadmap.
These rounds of consultation included both targeted requests for feedback to selected stakeholders and the current public consultation. The contributions will be published on the AIGHD website (in cases consent was provided) and will be used to complete the final version of the Roadmap.
Draft Roadmap and online form
Draft global roadmap for research and development for tuberculosis vaccines (PDF)
Background document which summarizes the state-of-the-art in research and development for new vaccines for tuberculosis for a TB Vaccine Research & Development Roadmap (PDF).
Contribute to this consultation by filling in the on-line survey.
Emory Vaccine Center [to 16 Jan 2021]
http://www.vaccines.emory.edu/
Vaccine Center News
No new digest content identified.
European Medicines Agency [to 16 Jan 2021]
http://www.ema.europa.eu/ema/
News & Press Releases
Meeting highlights from the Pharmacovigilance Risk Assessment Committee (PRAC) 11-14 January 2021
News 15/01/2021
First monthly summary safety report for COVID-19 vaccine Comirnaty
[See COVID – EMA above for detail]
News: EMA receives application for conditional marketing authorisation of COVID-19 Vaccine AstraZeneca
Last updated: 12/01/2021
European Vaccine Initiative [to 16 Jan 2021]
http://www.euvaccine.eu/
Latest News
McKinsey & Company awarded subcontract by EVI
13 Jan 2021
The European Vaccine Initiative (EVI) has selected McKinsey & Company to develop a business plan, including suitable governance structure and financing strategy, that can guide the establishment of a sustainable infrastructure for European vaccine research. Subcontracting McKinsey & Company adds valuable business experience and will be advantageous to the conceptual and technical design of a European vaccine infrastructure. The sub-contract is awarded as part of the project TRANSVAC-DS, Design study for a European vaccine infrastructure, which is funded by the European Commission and comprises a consortium of twenty-five partners from eleven European countries and includes leading academic and other organisations working in areas related to vaccine development…
FDA [to 16 Jan 2021]
https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/default.htm
Press Announcements /Selected Details
January 15, 2021 – Coronavirus (COVID-19) Update: January 15, 2021
:: Today, the FDA issued and immediately implemented a new guidance entitled, “Protecting Participants in Bioequivalence Studies for Abbreviated New Drug Applications During the COVID-19 Public Health Emergency.” This guidance assists prospective applicants of abbreviated new drug applications (ANDAs) on ensuring participants are protected when resuming or initiating bioequivalence studies conducted to support the approval of an ANDA that has been disrupted during the COVID-19 public health emergency.
January 12, 2021 – FDA Releases Artificial Intelligence/Machine Learning Action Plan
“This action plan outlines the FDA’s next steps towards furthering oversight for AI/ML-based SaMD,” said Bakul Patel, director of the Digital Health Center of Excellence in the Center for Devices and Radiological Health (CDRH). “The plan outlines a holistic approach based on total product lifecycle oversight to further the enormous potential that these technologies have to improve patient care while delivering safe and effective software functionality that improves the quality of care that patients receive. To stay current and address patient safety and improve access to these promising technologies, we anticipate that this action plan will continue to evolve over time.”
FDA – COVID-19 Vaccines [to 16 Jan 2021]
www.fda.gov/covid19vaccines
News and Updates; Upcoming Events
I’m a disabled woman of color. Here’s how I overcame my fear of receiving a COVID vaccineExternal Link Disclaimer
An FDA staff member discusses how her former hesitancy and fear grew into hope and a willingness to receive a COVID-19 vaccine.
01/14/2021
Fondation Merieux [to 16 Jan 2021]
http://www.fondation-merieux.org/
News, Events
Mérieux Foundation co-organized event
ADVA – Managing the severely ill dengue patients: adults & children
January 20, 2021 – 12:00pm – 3:30pm (CET) Webinar
Register
Context
Co-organized by Asia Dengue Voice & Action Group (ADVA), GDAC, Mérieux Foundation, ISNTD and SEAMEO in support of the 5th Asia Dengue Summit, this webinar is the first in a series titled “Decimate Dengue: The 5th ADS Pre-Summit Webinars”, aimed at bridging the dengue healthcare and research communities via an online platform that allows for closer interaction, learning and adoption of the latest dengue management strategies in the run-up to the 5th Asia Dengue Summit 2022.
Gavi [to 16 Jan 2021]
https://www.gavi.org/
News releases
500,000 doses of Ebola vaccine to be made available to countries for outbreak response
12 Jan 2021
:: A Gavi-funded global emergency stockpile of Ebola vaccines will be accessible to all countries following a procurement process concluded by UNICEF
:: Seth Berkley: “The accelerated development of the Ebola vaccine was possible thanks to a first-of-its-kind agreement between Gavi and the vaccine manufacturer, which set a precedent for fast-tracking development and production of vaccines against COVID-19”
:: The new Ebola vaccine played an important role in ending the Ebola outbreak in the eastern part of the Democratic Republic of the Congo (DRC), which was officially declared over in June last year. The stockpile is an example of how Gavi, the Vaccine Alliance and UNICEF are strengthening the world’s defenses against potentially devastating infectious disease outbreaks
GHIT Fund [to 16 Jan 2021]
https://www.ghitfund.org/newsroom/press
GHIT was set up in 212 with the aim of developing new tools to tackle infectious diseases that
Press Releases
No new digest content identified.
Global Fund [to 16 Jan 2021]
https://www.theglobalfund.org/en/news/
News
Global Fund signs a record-breaking $8.54 billion in grants to fight HIV, TB and malaria
12 January 2021
In 2020, the Global Fund signed 157 grants for a total of US$8.54 billion for lifesaving HIV, TB and malaria programs and to strengthen systems for health. This is the highest amount of grants ever signed in a single year by the Global Fund. The grants will begin implementation this month.
Global Research Collaboration for Infectious Disease Preparedness [GloPID-R] [to 16 Jan 2021]
https://www.glopid-r.org/news/
News
No new digest content identified.
Hilleman Laboratories [to 16 Jan 2021]
http://www.hillemanlabs.org/
No new digest content identified.
Human Vaccines Project [to 16 Jan 2021]
http://www.humanvaccinesproject.org/media/press-releases/
Press Releases
HVP COVID Report
Issue 24: Can Changing COVID Vaccine Regimens End the Pandemic Faster?
Jan 15, 2021
…Recently, there was also discussion among U.S. scientists and the company Moderna about cutting the two doses of their mRNA vaccine in half for people ages 18-55 in an effort to double the available doses. This suggestion was based on data from earlier stage clinical trials of this vaccine.
For now, the U.S. Food and Drug Administration (FDA) is opposed to any changes to the dosing regimens. In a statement issued on January 4th, the agency, which granted an Emergency Use Authorization to both the Pfizer/BioNtech and Moderna mRNA COVID-19 vaccines in December, said that altering the dose, reducing the number of doses, or extending the time between doses of these vaccines are reasonable questions to consider and evaluate in clinical trials. “However, at this time, suggesting changes to the FDA-authorized dosing or schedules of these vaccines is premature and not rooted solidly in available evidence.” The AstraZeneca vaccine has not been submitted to the FDA yet for authorization, however, it was authorized already in the U.K., India, and other countries.
But others argue that these strategies to free up more vaccine are warranted given the terrifying course of the pandemic. “As long as there is a shortage of vaccine, protection of the largest number of people against death and disability in the shortest time should take priority over formalism and insufficiency of conclusive data,” says Stanley Plotkin, veteran vaccine developer, Emeritus Professor of Pediatrics at the University of Pennsylvania, and founding board chair of the Human Vaccines Project. “This is an emergency, not a time to stick to routine rules.”
Furthermore, Plotkin argues that there is actually an immunological benefit to delaying vaccine doses. “The optional delay for non-live vaccines is four to six months,” he says…
IAVI [to 16 Jan 2021]
https://www.iavi.org/newsroom
PRESS RELEASES/FEATURES
No new digest content identified.
International Coalition of Medicines Regulatory Authorities [ICMRA]
http://www.icmra.info/drupal/en/news
Selected Statements, Press Releases, Research
No new digest content identified.
International Generic and Biosimilar Medicines Association [IGBA]
https://www.igbamedicines.org/
News
Sudarshan Jain takes over the position of IGBA Chair (January 2021)
Geneva, 11 January 2021 – IGBA, the International Generic and Biosimilar Medicines Association (IGBA), representing global manufacturers of generic and biosimilar medicines, announced today that Sudarshan Jain, Secretary General of the Indian Pharmaceutical Alliance (IPA), is taking over the position of IGBA Chair for 2021 from Hanan Sboul, Secretary General of the Jordanian Association of Pharmaceutical Manufacturers.
IFFIm
http://www.iffim.org/
Press Releases/Announcements
No new digest content identified.
IFRC [to 16 Jan 2021]
http://media.ifrc.org/ifrc/news/press-releases/
Selected Press Releases, Announcements
Americas, Honduras
Red Cross: Providing services and protection to migrants in Central America is a humanitarian imperative
Panama/Geneva, 15 January 2021 – The Red Cross is preparing to provide humanitarian assistance to migrants ready to depart Honduras for Guatemala as part of a ‘migrant caravan’. More than 4,000 thousand people are expected to join the caravan that will …
15 January 2021
Armenia, Azerbaijan, Europe, Georgia
New study finds coronavirus has left older people poorer, sicker and more alone
Budapest/Geneva, 13 January 2021 – The COVID-19 pandemic is having catastrophic health, social and financial impacts on older people in Europe’s South Caucasus region, according to a new study led by the International Federation of Red Cross and Red Cr …
13 January 2021
Global
UNICEF, WHO, IFRC and MSF announce the establishment of a global Ebola vaccine stockpile
NEW YORK/ GENEVA, 12 JANUARY 2021: The four leading international health and humanitarian organizations announced today the establishment of a global Ebola vaccine stockpile to ensure outbreak response. The effort to establish the stockpile was led by …
12 January 2021
Global
COVID-19: Vaccines alone will not end pandemic, warns IFRC
Geneva, 11 January 2021 – With COVID-19 vaccines rolling out across many countries, the world’s largest humanitarian network is once again warning that vaccines alone will not end the pandemic. People need to remain vigilant and continue to adhere to b …
… Emanuele Capobianco, IFRC’s Director of Health, said: “We are very concerned about this convergence of a potential false sense of security due to the rollout of vaccines, the emergence of new variants, and the impact of holiday-season travel. Our first line of defence against the virus remains our individual behaviour. Beyond this, the ability of Governments to take swift actions based on scientific evidence is also key to slowing down the pandemic. “Vaccines will help, but unless we all remain vigilant, and unless their deployment is accelerated across the world in a fair and equitable manner, the entire world remains at risk.”
Institut Pasteur [to 16 Jan 2021]
https://www.pasteur.fr/en/press-area
Press documents
No new digest content identified.
IRC International Rescue Committee [to 16 Jan 2021]
http://www.rescue.org/press-release-index
Media highlights [Selected]
Press Release
Billions will not receive a COVID-19 vaccine in 2021
January 12, 2021
:: Shortages of COVID-19 vaccines, coupled with vaccine nationalism, means the majority of people in crisis and conflict-affected contexts will not receive a COVID-19 vaccine this year.
:: Investments must be made immediately to strengthen health systems, including shoring up supply chains, allocating sustainable and adequate financing, and empowering community and frontline health workers to ensure no one is left behind.
:: Efforts must also be focused on continuing routine immunizations – in addition to preparation for the COVID-19 vaccines – to protect hard-won gains and avoid the spread of other vaccine-preventable disease outbreaks.
Press Release
24 million Yemenis at catastrophic humanitarian risk following new U.S. terrorist designations of Ansar Allah, warns IRC
January 11, 2021
IVAC [to 16 Jan 2021]
https://www.jhsph.edu/research/centers-and-institutes/ivac/index.html
Updates; Events
No new digest content identified.
IVI [to 16 Jan 2021]
http://www.ivi.int/
Selected IVI News, Announcements, Events
No new digest content identified.
JEE Alliance [to 16 Jan 2021]
https://www.jeealliance.org/
Selected News and Events
No new digest content identified.
Johns Hopkins Center for Health Security [to 16 Jan 2021]
https://www.centerforhealthsecurity.org/news/center-news/
Center News
No new digest content identified.
MSF/Médecins Sans Frontières [to 16 Jan 2021]
http://www.msf.org/
Latest [Selected Announcements]
CENTRAL AFRICAN REPUBLIC
MSF teams ramp up support as violence escalates
PROJECT UPDATE14 JAN 2021
ETHIOPIA
Providing assistance to people in Ethiopia and Sudan in wake of Tigray violence
PROJECT UPDATE12 JAN 2021
SWITZERLAND
Responding to the second wave of COVID-19 in Switzerland
PROJECT UPDATE11 JAN 2021
National Vaccine Program Office – U.S. HHS [to 16 Jan 2021]
https://www.hhs.gov/vaccines/about/index.html
Upcoming Meetings/Latest Updates
No new digest content identified.
NIH [to 16 Jan 2021]
http://www.nih.gov/news-events/news-releases
News Releases
NIH scientists identify nutrient that helps prevent bacterial infection
January 15, 2021 — Taurine, which helps the body digest fats and oils, could offer treatment benefit.
PATH [to 16 Jan 2021]
https://www.path.org/media-center/
Press Release
No new digest content identified
Sabin Vaccine Institute [to 16 Jan 2021]
http://www.sabin.org/updates/pressreleases
Statements and Press Releases
Sabin Vaccine Institute Funds Researchers to Investigate COVID-19 Misinformation, Design Solutions to Increase Vaccine Acceptance
Wednesday, January 13, 2021
, D.C. – The Sabin Vaccine Institute (Sabin) announced today that it has awarded grants to research teams in four countries to explore the social drivers of COVID-19 misinformation, and its impact on routine immunization acceptance and the acceptance of a COVID-19 vaccine.
The grants are part of Sabin’s Social and Behavioral Interventions for Vaccination Acceptance Small Grants Program, which provides funding to researchers in low- and middle-income countries to better understand the social drivers of vaccination and design small-scale interventions to assess their impact on vaccination acceptance.
Five research teams in India, Kenya, Pakistan and Uganda will receive up to $30,000 to conduct this research and pilot a small-scale intervention in their respective communities over a period of 10 months…
UNAIDS [to 16 Jan 2021]
http://www.unaids.org/en
Selected Press Releases/Reports/Statements
12 January 2021
Vulnerability mapping to help sex workers in Bangladesh and Myanmar
11 January 2021
Attaining UNAIDS’ proposed societal and legal barrier targets could stop 440 000 AIDS-related deaths
UNICEF [to 16 Jan 2021]
https://www.unicef.org/media/press-releases
Selected Press releases, Statements
Statement 01/12/2021
Children cannot afford another year of school disruption
Statement by UNICEF Executive Director Henrietta Fore
Press release 01/11/2021
UNICEF, WHO, IFRC and MSF announce the establishment of a global Ebola vaccine stockpile
[See Milestones above for detail]
Unitaid [to 16 Jan 2021]
https://unitaid.org/
Featured News
12 JANUARY 2021
Unitaid funding sees launch of world’s first long-acting medicines centre at University of Liverpool
Geneva – Efforts to revolutionise treatments for debilitating infectious diseases have been amplified today with the launch of a new research centre at the University of Liverpool.
Established as part of a US$40 million international research consortium, primarily funded by Unitaid, the University of Liverpool’s Centre of Excellence for Long-acting Therapeutics (CELT) will be the first of its kind in the world.
By repurposing existing medicines into slow-release formulations, where drug effectiveness can be sustained over several months, ‘long-acting’ technology has already been successfully implemented in the fields of contraception and schizophrenia.
It now has the potential to improve the outcomes for treatment and prevention of deadly diseases such as HIV, malaria, Hepatitis C and tuberculosis, which particularly impact low- and middle-income countries.
Current treatment courses for these conditions have often resulted in poor outcomes in low-resource environments, as those living with diseases struggle with regimens that can involve taking dozens of tablets every day and rely on regular access to healthcare settings.
CELT’s mission is to broaden knowledge of long-acting medicines and disseminate key research, with the aim of revolutionising how these devastating diseases are treated, particularly in countries where access to healthcare is challenging…
Vaccination Acceptance Research Network (VARN) [to 16 Jan 2021]
https://vaccineacceptance.org/news.html#header1-2r
Announcements
No new digest content identified.
Vaccine Confidence Project [to 16 Jan 2021]
http://www.vaccineconfidence.org/
News, Research and Reports
15 Jan 2021
CONVINCE (COVID New Vaccine Information, Communication and Engagement)
Vaccine Literacy: Building Public Support for a COVID-19 vaccine
Call to Action from Wilton Park, City University of New York (CUNY) Graduate School of Public Health & Health Policy and the Vaccine Confidence Project at the London School of Hygiene and Tropical Medicine
Effective communication to prepare a ‘vaccine literate’ public and persuade policymakers to trust the science and embrace new vaccines for COVID-19 as they become available must begin now.
To ensure a COVID-19 vaccine is accepted, we must invest in:
:: a multi-sectoral commitment to develop trust in vaccines
:: preparation of accurate and convincing information sources about vaccines
:: innovative multi-media and interpersonal approaches to communicate and engage with all audiences..
Vaccine Education Center – Children’s Hospital of Philadelphia [to 16 Jan 2021]
http://www.chop.edu/centers-programs/vaccine-education-center
News
No new digest content identified.
Wellcome Trust [to 16 Jan 2021]
https://wellcome.ac.uk/news
News
Explainer | 13 January 2021
What drugs are working as treatments for Covid-19?
From existing antivirals to new antibody therapies – researchers are working at incredible speed to find the best drugs to treat Covid-19.
The Wistar Institute [to 16 Jan 2021]
https://www.wistar.org/news/press-releases
Press Releases
Jan. 13, 2021
New Insights Into the Control of Inflammation
The EGR1 transcription factor has distinct roles in early and late macrophage maturation stages, blunting macrophage activation and inflammation.
Press Release
Jan. 12, 2021
Wistar Researchers Develop New Humanized Mouse Model That Provides Insight Into Immunotherapy Resistance
Tumor-infiltrating mast cells are connected to immune checkpoint inhibitor resistance in melanoma.
Press Release
Jan. 11, 2021
Wistar Scientists Discover Link Between a Genetic Driver of Ovarian Cancer and Metabolism, Opening the Way for New Therapeutic Strategies
Loss of ARID1A causes increased glutamine metabolism, which can be blocked pharmacologically to target ARID1A-mutant tumors.
WFPHA: World Federation of Public Health Associations [to 16 Jan 2021]
https://www.wfpha.org/
Latest News
Norwegian Perspectives on Immunization: A Positive Response to Public Health Advocacy during COVID-19
Jan 14, 2021
Throughout the COVID-19 pandemic, health inequity has become increasingly apparent. The public health workforce has rapidly mobilized to advocate…
Building Confidence in COVID-19 Vaccination
Jan 13, 2021
The COVID-19 vaccines bring the promise of a global rescue from the coronavirus pandemic; however, building people’s confidence in these new vaccines seems to be a herculean task. Myths and misinformation…
World Organisation for Animal Health (OIE) [to 16 Jan 2021]
https://www.oie.int/en/for-the-media/press-releases/2021/
No new digest content identified.
::::::
ARM [Alliance for Regenerative Medicine] [to 16 Jan 2021]
Press Releases – Alliance for Regenerative Medicine (alliancerm.org)
Press Releases
No new digest content identified.
BIO [to 16 Jan 2021]
https://www.bio.org/press-releases
Press Releases
BIO Releases Statement Concerning Future Political Giving
January 11, 2021
Dr. Michelle McMurry-Heath, president and CEO of the Biotechnology Innovation Organization (BIO), released the following statement on BIO’s political contributions: “As of today BIO will be pausing our political giving so we can…
DCVMN – Developing Country Vaccine Manufacturers Network [to 16 Jan 2021]
http://www.dcvmn.org/
News; Upcoming events
No new digest content identified.
ICBA – International Council of Biotechnology Associations [to 16 Jan 2021]
https://internationalbiotech.org/news/
News
No new digest content identified.
IFPMA [to 16 Jan 2021]
http://www.ifpma.org/resources/news-releases/
Selected Press Releases, Statements, Publications
Innovative biopharmaceutical industry comment on COVID-19 vaccines dosing strategies and recommend following the science
13 January 2021
[See COVID above for full text]
Speak Up Africa and IFPMA start their search for winners of the Africa Young Innovators
12 January 2021
Pharma innovation delivers COVID-19 solutions beyond expectations, but calls for the dilution of intellectual property
08 December 2020
…despite a lack of any evidence, there have been claims that intellectual property (IP) rights are hindering the response to the pandemic. A waiver proposal currently being discussed by the World Trade Organization (WTO) is looking to suspend Member States’ obligations to protect innovator’s intellectual property assets that are critical to tackle the COVID-19 pandemic. In view of the progress made so far in providing COVID-19 solutions and the partnerships in place to boost research and scale-up manufacturing of vaccines and treatments, diluting national and international IP frameworks during this pandemic is counterproductive. It will not lead to faster research and development or access, but it will undermine confidence in what has proven to be a well-functioning IP system, allowing industry to partner with confidence with academia, research institutes, foundations and other private companies, significantly expediting the research and development of medicines to address the worlds’ many unmet medical needs…
PhRMA [to 16 Jan 2021]
http://www.phrma.org/
Selected Press Releases, Statements
Innovative Biopharmaceutical Industry Comment on COVID-19 Vaccines Dosing Strategies and Recommend Following the Science
January 13, 2021
This statement on COVID-19 vaccine dosing strategies was issued by the Pharmaceutical Research and Manufacturers of America (PhRMA), the International Federation of Pharmaceutical Manufacturers & Associations (IFPMA), the International Council of Biotech Associations (ICBA), the Biotechnology Innovation Organization (BIO), the European Federation of Pharmaceutical Industries and Associations (EFPIA) and Vaccines Europe.
PRESS RELEASE
[See COVID above for full text]
PhRMA Statement on Political Giving
January 12, 2021
Pharmaceutical Research and Manufacturers of America (PhRMA) president and CEO Stephen J. Ubl released the following statement regarding violent protests in Washington D.C. and the organization’s decision on political giving.
PRESS RELEASE
Paper finds gaps in health data are a barrier to health equity
January 11, 2021
COVID-19 has laid bare racial disparities in the United States health system. For example, COVID-19 cases among Native Hawaiian/Pacific Islanders are up to 2.5 times higher as compared to whites. Similarly, the rate of COVID-19 related deaths among Black Americans and American Indian/Alaskan Natives are twice as high as whites. A recent PhRMA paper finds that gaps in information on race, ethnicity and health are barriers to achieving health equity for many groups…
PhRMA has decided to address these issues head on and will support others willing to do the same. Read our first paper in the series, “Gaps in Available Data Exacerbate Health Disparities and Create Barriers to Change.”
Emily Donald
BLOG POST
Journal Watch
Vaccines and Global Health: The Week in Review continues its weekly scanning of key peer-reviewed journals to identify and cite articles, commentary and editorials, books reviews and other content supporting our focu-s on vaccine ethics and policy. Journal Watch is not intended to be exhaustive, but indicative of themes and issues the Center is actively tracking. We selectively provide full text of some editorial and comment articles that are specifically relevant to our work. Successful access to some of the links provided may require subscription or other access arrangement unique to the publisher.
If you would like to suggest other journal titles to include in this service, please contact David Curry at: david.r.curry@centerforvaccineethicsandpolicy.org
American Journal of Public Health
February 2021 111(2)
http://ajph.aphapublications.org/toc/ajph/current
PERSPECTIVES
The Need for Novel Approaches in Assessing the Value of COVID-19 Vaccines
Immunization/Vaccines, Social Science, Socioeconomic Factors, Statistics/Evaluation/Research, Health Policy, Other Statistics/Evaluation/Research
Aris Angelis, Rob Baltussen and Tommi Tervonen
111(2), pp. 205–208
Health Affairs
Vol. 40, No. 1 January 2021
https://www.healthaffairs.org/toc/hlthaff/current
COVID-19 Response, Medicaid & More
Analysis
An Overview Of Vaccine Development, Approval, And Regulation, With Implications For COVID-19
Aaron S. Kesselheim, Jonathan J. Darrow, Martin Kulldorff, Beatrice L. Brown, Mayookha Mitra-Majumdar, ChangWon C. Lee, Osman Moneer, and Jerry Avorn
Abstract
The Food and Drug Administration (FDA) approves vaccines when their benefits outweigh the risks for their intended use. In this article we review the standard FDA approach to vaccine evaluation, which underpins its current approaches to assessment of vaccines to prevent coronavirus disease 2019 (COVID-19). The FDA has established pathways to accelerate vaccine availability before approval, such as Emergency Use Authorization, and to channel resources to high-priority products and allow more flexibility in the evidence required for approval, including accelerated approval based on surrogate markers of effectiveness. Among the thirty-five new vaccines approved in the US from 2006 through October 2020, about two-thirds of their pivotal trials used the surrogate outcome of immune system response, and only one-third evaluated actual disease incidence. Postapproval safety surveillance of new vaccines, and particularly vaccines receiving expedited approval, is crucial. This has generally been accomplished through such mechanisms as the Centers for Disease Control and Prevention (CDC) and FDA Vaccine Adverse Event Reporting System, the CDC Vaccine Safety Datalink, and the CDC Clinical Immunization Safety Assessment Project. Adverse events detected in this way may lead to changes in a vaccine’s recommended use or withdrawal from the market. Regulatory oversight of new vaccines must balance speed with rigor to effectively address the pandemic.
Health Affairs
Vol. 40, No. 1 January 2021
https://www.healthaffairs.org/toc/hlthaff/current
Analysis COVID-19
COVID-19 Vaccine To Vaccination: Why Leaders Must Invest In Delivery Strategies Now
Rebecca L. Weintraub, Laura Subramanian, Ami Karlage, Iman Ahmad, and Julie Rosenberg
Free Access
Abstract
Worldwide, leaders are implementing nonpharmaceutical interventions to slow transmission of the novel coronavirus while pursuing vaccines that confer immunity to severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) infection. In this article we describe lessons learned from past pandemics and vaccine campaigns about the path to successful vaccine delivery. The historical record suggests that to have a widely immunized population, leaders must invest in evidence-based vaccine delivery strategies that generate demand, allocate and distribute vaccines, and verify coverage. To generate demand, there must be an understanding of the roots of vaccine hesitancy, involvement of trusted sources of authority in advocacy for vaccination, and commitment to longitudinal engagement with communities. To allocate vaccines, qualified organizations and expert coalitions must be allowed to determine evidence-based vaccination approaches and generate the political will to ensure the cooperation of local and national governments. To distribute vaccines, the people and organizations with expertise in manufacturing, supply chains, and last-mile distribution must be positioned to direct efforts. To verify vaccine coverage, vaccination tracking systems that are portable, interoperable, and secure must be identified. Lessons of past pandemics suggest that nations should invest in evidence-informed strategies to ensure that coronavirus disease 2019 (COVID-19) vaccines protect individuals, suppress transmission, and minimize disruption to health services and livelihoods.
Health Affairs
Vol. 40, No. 1 January 2021
https://www.healthaffairs.org/toc/hlthaff/current
Research Article COVID-19
Clinical Outcomes Of A COVID-19 Vaccine: Implementation Over Efficacy
A. David Paltiel, Jason L. Schwartz, Amy Zheng, and Rochelle P. Walensky
Free Access
Abstract
The global effort to develop a coronavirus disease 2019 (COVID-19) vaccine is on track to produce one or more authorized vaccines. We examine how different definitions and thresholds of vaccine efficacy, coupled with different levels of implementation effectiveness and background epidemic severity, translate into outcomes including cumulative infections, hospitalizations, and deaths. Using a mathematical simulation of vaccination, we find that factors related to implementation will contribute more to the success of vaccination programs than a vaccine’s efficacy as determined in clinical trials. The benefits of a vaccine will decline substantially in the event of manufacturing or deployment delays, significant vaccine hesitancy, or greater epidemic severity. Our findings demonstrate the urgent need for health officials to invest greater financial resources and attention to vaccine production and distribution programs, to redouble efforts to promote public confidence in COVID-19 vaccines, and to encourage continued adherence to other mitigation approaches, even after a vaccine becomes available.
Health Affairs
Vol. 40, No. 1 January 2021
https://www.healthaffairs.org/toc/hlthaff/current
Analysis COVID-19
Consideration Of Value-Based Pricing For Treatments And Vaccines Is Important, Even In The COVID-19 Pandemic
Peter J. Neumann, Joshua T. Cohen, David D. Kim, and Daniel A. Ollendorf
Free Access
Abstract
Prices send signals about consumer preferences and thus stimulate producers to make more of what people want. Pricing in a pandemic is complicated and fraught. The policy puzzle involves balancing lower prices to ensure access to essential medications, vaccines, and tests against the need for adequate revenue streams to provide manufacturers with incentives to make the substantial, risky investments needed to develop products in the first place. We review alternative pricing strategies (cost recovery models, monetary prizes, and advance market commitments) for coronavirus disease 2019 (COVID-19) drugs, vaccines, and diagnostics. Hybrid pricing strategies are undoubtedly needed in a pandemic, but even in a public health crisis, value-based pricing is important. Cost-effectiveness analyses can inform pricing. Ideally, analyses would be conducted from both a health system and a societal perspective. Incorporating the added value of social benefits into cost-effectiveness analyses does not mean that manufacturers should capture the entire societal benefit of a diagnostic, vaccine, or therapy. Such analyses can provide important information and help policy makers consider the full costs and benefits of products and the wide-ranging ramifications of their actions.
Health Affairs
Vol. 40, No. 1 January 2021
https://www.healthaffairs.org/toc/hlthaff/current
Analysis COVID-19
Ensuring Equitable Access To COVID-19 Vaccines In The US: Current System Challenges And Opportunities
Angela K. Shen, Richard Hughes IV, Erica DeWald, Sara Rosenbaum, Amy Pisani, and Walt Orenstein
Free Access
Abstract
There has been a worldwide effort to accelerate the development of safe and effective vaccines for severe acute respiratory syndrome coronavirus-2. When vaccines become licensed and available broadly to the public, the final hurdle is equitable distribution and access for all who are recommended for vaccination. Frameworks and existing systems for allocation, distribution, vaccination, and monitoring for safety and effectiveness are assets of the current immunization delivery system that should be leveraged to ensure the equitable distribution and broad uptake of licensed vaccines. The system should be strengthened to address gaps in access to immunization services and to modernize the public health infrastructure. We offer five recommendations as guideposts to ensure that policies and practices at the federal, state, local, and tribal levels support equity, transparency, accountability, availability, and access to coronavirus disease 2019 vaccines.