Organization Announcements
Editor’s Note:
Careful readers will note that the number and range of organizations now monitored in our Announcements section below has grown as the impacts of the pandemic have spread across global economies, supply chains and programmatic activity of multilateral agencies and INGOs.
Paul G. Allen Frontiers Group [to 26 Jun 2021]
https://alleninstitute.org/what-we-do/frontiers-group/news-press/
News
News from The Paul G. Allen Frontiers Group: June 2021
June 23, 2021
Highlights include a new study by the Allen Discovery Center for Human Brain Evolution, a Science in Sixty video featuring Kathryn Richmond, and more.
BARDA – U.S. Department of HHS [to 26 Jun 2021]
https://www.phe.gov/about/barda/Pages/default.aspx
News
No new digest content identified.
BMGF – Gates Foundation [to 26 Jun 2021]
https://www.gatesfoundation.org/ideas/media-center
Press Releases and Statements
No new digest content identified.
Bill & Melinda Gates Medical Research Institute [to 26 Jun 2021]
https://www.gatesmri.org/
The Bill & Melinda Gates Medical Research Institute is a non-profit biotech organization. Our mission is to develop products to fight malaria, tuberculosis, and diarrheal diseases—three major causes of mortality, poverty, and inequality in developing countries. The world has unprecedented scientific tools at its disposal; now is the time to use them to save the lives of the world’s poorest people
Jun 10, 2021
Penny Heaton @drpennyheaton
It’s a bittersweet day as I announce my departure from @GatesMRI to join @JanssenGlobal. I’m grateful for the opportunity to have led such an incredible organization – and I’m excited to lead a team transforming global #vaccine development.
CARB-X [to 26 Jun 2021]
https://carb-x.org/
News
No new digest content identified.
Center for Vaccine Ethics and Policy – GE2P2 Global Foundation [to 26 Jun 2021]
https://centerforvaccineethicsandpolicy.net/
News/Analysis/Statements
:: Past weekly editions and posting of all segments of Vaccines and Global Health: The Week in Review are available here.
:: [NEW] Informed Consent: A Monthly Review – June 2021 is now posted here
CEPI – Coalition for Epidemic Preparedness Innovations [to 26 Jun 2021]
http://cepi.net/
Latest News
No new digest content identified.
CIOMS – COUNCIL FOR INTERNATIONAL ORGANIZATIONS OF MEDICAL SCIENCES [to 26 Jun 2021]
https://cioms.ch/
Publications
CIOMS Cumulative Pharmacovigilance Glossary (Version 1.1)
Year of publication: 2021 Number of pages: 81
PDF: https://cioms.ch/wp-content/uploads/2021/03/CIOMS-Cumulative-Glossary_v1.1_3Jun2021.pdf
This glossary compiles the terms and definitions from published CIOMS pharmacovigilance reports.
We welcome all feedback. Please e-mail your recommendations to info@cioms.ch.
Clinical research in resource-limited settings
CIOMS
ISBN: 978-929036100-8
Year of publication: 2021 Number of pages: 136
PDF: https://cioms.ch/wp-content/uploads/2021/06/CIOMS_ClinicalResearch_RLS.pdf
Description
Evidence generated through responsible clinical research is one of the major pillars of the advancement of health care. In past decades there has been tremendous progress in the clinical research and development (R & D) environment globally, with increasing attention being paid to the health needs of people in resource-limited settings, where most of the preventable morbidity and mortality occurs. However, financial, social, ethical and regulatory challenges persist in low- and middle-income countries (LMICs), and most clinical research today is still being conducted in and for high-income countries (HICs). The aim of this report is to provide balanced arguments to promote scientifically sound good quality clinical research in low-resource settings.
The Council for International Organizations of Medical Sciences (CIOMS) is an international, non-governmental, non-profit organization with the mission to advance public health through guidance on health research and policy including ethics, medical product development and safety. This report reflects the consensus opinion of the CIOMS Working Group on Clinical Research in Resource-Limited Settings, and was finalized in line with comments received during public consultation. The report is intended for governments and regulatory authorities, the research community and sponsors, as well as international organizations involved in funding or conducting research. The report provides a comprehensive set of recommendations to all major stakeholders. While it builds on the 2016 CIOMS International Ethical Guidelines for Health-related Research Involving Humans, it is not intended to supersede those guidelines.
DARPA – Defense Advanced Research Projects Agency [to 26 Jun 2021
https://www.darpa.mil/news
News
No new digest content identified.
Duke Global Health Innovation Center [to 26 Jun 2021]
https://dukeghic.org/
Friday, June 18, 2021
The G7 underwhelms, with numbers that don’t add up
The G7 began their much-anticipated summit last week with a target to reach 1 billion doses of Covid-19 vaccine in pledged donations for low- and middle-inco
EDCTP [to 26 Jun 2021]
http://www.edctp.org/
The European & Developing Countries Clinical Trials Partnership (EDCTP) aims to accelerate the development of new or improved drugs, vaccines, microbicides and diagnostics against HIV/AIDS, tuberculosis and malaria as well as other poverty-related and neglected infectious diseases in sub-Saharan Africa, with a focus on phase II and III clinical trials
No new digest content identified.
Emory Vaccine Center [to 26 Jun 2021]
http://www.vaccines.emory.edu/
Vaccine Center News
No new digest content identified.
European Vaccine Initiative [to 26 Jun 2021]
http://www.euvaccine.eu/
Latest News
EVI signs MoU with CEPI
21 June 2021
The European Vaccine Initiative (EVI), as coordinator of the TRANSVAC2 vaccine research infrastructure, and the Coalition for Epidemic Preparedness Innovations (CEPI) recently signed a Memorandum of Understanding (MoU), laying the foundation to establish a vaccinology training collaboration with the TRANSVAC2 project.
TRANSVAC2 is dedicated to accelerating and improving vaccine development, by strengthening and disseminating European vaccine expertise. As one of the support activities offered to the scientific community, TRANSVAC2 provides training courses surrounding fundamental and advanced knowledge on a wide-range of vaccine development-related topics. With this MoU, we will boost access of advanced vaccine research and development training to CEPI’s team members, thereby directly supporting CEPI´s important mission to accelerate the development of vaccines against emerging infectious diseases.
EVI welcomes this new partnership agreement with CEPI that attests to the high quality and demand of trainings and courses organised by TRANSVAC2 partners, including University of Oxford, Vaccine Formulation Institute, Wageningen University, Biomedical Primate Research Centre (BPRC), The French Alternative Energies and Atomic Energy Commission (CEA), Fraunhofer (IMI-Fraunhofer), University of Siena (UNISI) and EATRIS.
FDA [to 26 Jun 2021]
https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/default.htm
Press Announcements /Selected Details
June 25, 2021 – Coronavirus (COVID-19) Update: June 25, 2021
Today, the FDA is announcing revisions to the patient and provider fact sheets for the Moderna and Pfizer-BioNTech COVID-19 vaccines regarding the suggested increased risks of myocarditis (inflammation of the heart muscle) and pericarditis (inflammation of the tissue surrounding the heart) following vaccination. For each vaccine, the Fact Sheet for Healthcare Providers Administering Vaccine (Vaccination Providers) has been revised to include a warning about myocarditis and pericarditis and the Fact Sheet for Recipients and Caregivers has been revised to include information about myocarditis and pericarditis. This update follows an extensive review of information and the discussion by CDC’s Advisory Committee on Immunization Practices meeting on Wednesday. The data presented at this meeting reinforced the FDA’s decision to revise the fact sheets and further informed the specific revisions. The warning in the Fact Sheets for Healthcare Providers Administering Vaccines notes that reports of adverse events suggest increased risks of myocarditis and pericarditis, particularly following the second dose and with onset of symptoms within a few days after vaccination. Additionally, the Fact Sheets for Recipients and Caregivers for these vaccines note that vaccine recipients should seek medical attention right away if they have chest pain, shortness of breath, or feelings of having a fast-beating, fluttering, or pounding heart after vaccination. The FDA and CDC are monitoring the reports, collecting more information, and will follow-up to assess longer-term outcomes over several months.
June 24, 2021 – Coronavirus (COVID-19) Update: FDA Authorizes Drug for Treatment of COVID-19
Today, the U.S. Food and Drug Administration issued an emergency use authorization (EUA) for the drug Actemra (tocilizumab) for the treatment of hospitalized adults and pediatric patients (2 years of age and older) who are receiving systemic corticosteroids and require supplemental oxygen, non-invasive or invasive mechanical ventilation, or extracorporeal membrane oxygenation (ECMO). Actemra is not authorized for use in outpatients with COVID-19.
June 22, 2021 – Coronavirus (COVID-19) Update: June 22, 2021
Fondation Merieux [to 26 Jun 2021]
http://www.fondation-merieux.org/
News, Events
No new digest content identified
Gavi [to 26 Jun 2021]
https://www.gavi.org/
News Releases
24 June 2021
Gavi Board strengthens commitment to reaching the most vulnerable through routine immunisation and COVAX
[See COVID above for full text]
23 June 2021
What does COVAX’s latest supply forecast tell us?
[See COVID above for full text]
24 June 2021
Gavi and the Global Fund sign groundbreaking agreement with International Federation of Accountants to support in-country financial management
Geneva, 24 June 2021 – Global health leaders Gavi, the Vaccine Alliance and the Global Fund to Fight AIDS, Tuberculosis and Malaria have joined forces with the International Federation of Accountants (IFAC) to contribute to, and support, the implementation of robust accounting practices in the public health sector and to improve overall financial management of donor funds by implementing countries.
Through this Memorandum of Understanding (MoU), Gavi, the Global Fund and IFAC seek to strengthen the expertise of accountancy and finance professionals and help close the gaps in accountancy skills in implementing countries, which can impact the reliability and effectiveness of managing and disbursing funds. The MoU builds on a 2011 agreement and aims to optimize the joint efforts of global health partners to maximize the performance of investments and support the sustainability of health programs.
“Gavi has cooperated with IFAC and national accounting organizations for a number of years to ensure sound financial management of the funding we provide to countries – this agreement allows us to go much further with a focus on the health sector, in leveraging the expertise of the accountancy profession to boost transparency, build local skills and capacity to improve overall accounting practices, and build a stronger ethical framework,” Anuradha Gupta, Deputy CEO of Gavi, commented. “That will ultimately bring economic and societal benefits to everyone.”
“Equipping our implementing partners with the right financial management skills is essential to maximize the impact of our investments and contributes to greater results in the fight against HIV, tuberculosis and malaria,” said Adda Faye, Chief Financial Officer at the Global Fund. “We are excited to join efforts with Gavi and IFAC to strengthen financial management, reporting, accountability, and transparency to better serve the societies and people in countries receiving Global Fund investments.”
“Robust and transparent accounting and reporting systems are the bedrock of strong public financial management and are thereby critical to the effectiveness and impact of Gavi and The Global Fund’s disbursement of lifesaving funds and resources,” said IFAC President Alan Johnson. “Leveraging our global network and accountancy expertise, this MoU underscores the unique value that IFAC and our member bodies bring to organizations with a shared interest in enhancing the accountancy function to build a resilient and sustainable public health sector that leads to a fairer society for all.”
This work will start with a number of pilot countries in collaboration with local professional accountancy organizations (PAOs). Gavi and the Global Fund will be responsible for funding, selecting beneficiary organizations, and monitoring the implementation of targeted capacity-building activities which include training, establishing and reinforcing accountancy standards, reinforcing ethics and whistle-blowing policies, implementing diversity and inclusiveness policies and helping set up appropriate legal foundations, governance structures, and operational capacity.
Ultimately, this partnership will lead to better integration of Gavi and the Global Fund investments into country systems, better internal controls to reduce fiduciary and financial risks, enhanced absorption of grants and ultimately greater impact.
Gavi allocates US$ 60 million for RBC GAM impact investment bond
:: Gavi is investing in the RBC Global Asset Management’s RBC Impact Bond Strategy, which supports measurable social and environmental progress
:: In recent years, Gavi has substantially expanded its embrace of sustainably focused investments
:: “Our partnership with RBC GAM puts Gavi’s investment resources where its values and mission are,” said Stephen Zinser, the Chair of Gavi’s investment committee
Geneva 21 June 2021 – Gavi, the Vaccine Alliance today announced it is allocating US$ 60 million to support economic development efforts with an aim of reducing poverty, promoting economic prosperity for all and protecting the planet.
In partnership with RBC Global Asset Management (RBC GAM), Gavi is investing in the RBC Impact Bond Strategy, which is dedicated to supporting measurable social and environmental progress through investments in projects like clean water and sanitation, good health and well-being, affordable housing, clean energy, economic equality, and quality education. This investment aims to deliver both competitive risk-adjusted returns and positive social and environmental outcomes.
“Our partnership with RBC GAM puts Gavi’s investment resources where its values and mission are,” said Stephen Zinser, the Chair of Gavi’s investment committee and CEO of Roxbury Asset Management…
GHIT Fund [to 26 Jun 2021]
https://www.ghitfund.org/newsroom/press
GHIT was set up in 212 with the aim of developing new tools to tackle infectious diseases that
No new digest content identified.
Global Fund [to 26 Jun 2021]
https://www.theglobalfund.org/en/news/
News & Stories
News
Gavi And The Global Fund Sign Groundbreaking Agreement With International Federation Of Accountants To Support In-Country Financial Management
24 June 2021
Global health leaders Gavi, the Vaccine Alliance and the Global Fund to Fight AIDS, Tuberculosis and Malaria have joined forces with the International Federation of Accountants (IFAC) to contribute to, and support, the implementation of robust accounting practices in the public health sector and to improve overall financial management of donor funds by implementing countries.
Global Research Collaboration for Infectious Disease Preparedness [GloPID-R] [to 26 Jun 2021]
https://www.glopid-r.org/news/
News
No new digest content identified.
Hilleman Laboratories [to 26 Jun 2021]
http://www.hillemanlabs.org/
Website reports “under maintenance” at inquiry
Human Vaccines Project [to 26 Jun 2021]
http://www.humanvaccinesproject.org/
No new digest content identified.
IAVI [to 26 Jun 2021]
https://www.iavi.org/newsroom
Latest News
No new digest content identified.
International Coalition of Medicines Regulatory Authorities [ICMRA]
http://www.icmra.info/drupal/en/news
Selected Statements, Press Releases, Research
No new digest content identified.
ICRC [to 26 Jun 2021]
https://www.icrc.org/en/whats-new
Selected News Releases, Statements, Reports
Sexual Violence, Conflict, and COVID-19: An Invisible Pandemic
Despite clear legal prohibitions, sexual violence remains widespread during armed conflicts and other situations of violence, as well as in detention. It occurs in various contexts and has grave humanitarian consequences.
24-06-2021 | Article
The challenges for humanitarian action in today’s conflicts: the perspective from the International Committee of the Red Cross
Excellencies, colleagues.It is a pleasure to address you today and I extend my warm appreciation to Minister of Defence Sergey Shoygu.
23-06-2021 | Statement
International Generic and Biosimilar Medicines Association [IGBA]
https://www.igbamedicines.org/
News
No new digest content identified.
IFFIm
http://www.iffim.org/
Press Releases/Announcements
No new digest content identified.
IFRC [to 26 Jun 2021]
http://media.ifrc.org/ifrc/news/press-releases/
Selected Press Releases, Announcements
Fiji
Pacific: Vaccination uptake critical as COVID-19 variants spread
Kuala Lumpur/Suva, 23 June 2021 – The International Federation of Red Cross and Red Crescent Societies (IFRC) urges all adults to get vaccinated in Pacific countries including Fiji as it sets grim national records for COVID-19 infections. Fiji is strug …
23 June 2021
Red Cross Red Crescent: Humanitarian sector joins forces to tackle ‘existential threat’ of climate change
Geneva, 22 June 2021 – The humanitarian sector has a key role to play in addressing the climate and environment crises that affect people’s lives and livelihoods around the world every day. This means walking the talk in terms of integrating climate sm …
22 June 2021
Fiji
Fiji TC Yasa: Six months on COVID-19 slows recovery efforts
Kuala Lumpur/Suva, 21 June 2021 – Six months after Cyclone Yasa tore through Fiji, leaving thousands of people homeless, essential movement restrictions to contain the dangerous spread of COVID-19 have delayed recovery efforts. Restrictions, while nece …
21 June 2021
Institut Pasteur [to 26 Jun 2021]
https://www.pasteur.fr/en/press-area
Press Info
Press release 22.06.2021
Drug repurposing to counter COVID-19: antiviral activity may be induced by phospholipidosis
Over the past 16 months and against the backdrop of the COVID-19 health crisis, thousands of drugs have been tested with a view to repurpose them to tackle SARS-CoV-2. Many of these drugs have demonstrated potential antiviral activity (23 compounds including hydroxychloroquine, azithromycin, amiodarone and 4 others tested in clinical trials). Given this high number of potential treatments, the University of California San Francisco (UCSF), the Quantitative Biosciences Institute, the Institut Pasteur and Novartis joined forces as part of an international collaboration to demonstrate that in vitro antiviral activity was in fact induced by a mechanism common to all the compounds: phospholipidosis. This research has revealed the importance of systematically testing phospholipidosis as part of the repurposing process, with a view to honing criteria for selecting drugs put forward for clinical trials. The results of the research were published in Science on June 22, 2021…
IOM / International Organization for Migration [to 26 Jun 2021]
http://www.iom.int/press-room/press-releases
News
UNAIDS, IOM: People on the Move Living with HIV Must Have Access to COVID-19 Vaccines
2021-06-24 23:51
Geneva – Migrants, refugees, internally displaced as well as crisis-affected and mobile populations who are living with HIV must have equitable access to COVID-19 vaccines, said the Joint United Nations Programme on HIV/AIDS (UNAIDS) and the International Organization for…
UN Agencies Welcome Relocation of 4,000 Vulnerable Asylum Seekers and Refugees from Greece, Encourage Further Steps Towards Predictable, Systematic European Mechanism
2021-06-24 22:36
Athens – IOM, the International Organization for Migration, UNHCR, the UN Refugee Agency, and UNICEF the UN Children’s Fund welcomed today (24-06) the relocation of 43 asylum seekers on two flights to France.
Nearly 14 Million Internally Displaced Persons, Refugees and Migrants Hit Hard by COVID-19 in East and Horn of Africa, New IOM-WFP Study Finds
2021-06-22 11:59
Nairobi – Nearly 9 million Internally Displaced Persons (IDPs), 4.7 million refugees and asylum-seekers, and hundreds of thousands of migrants in East and Horn of Africa are suffering some of the worst impacts of the COVID-19 pandemic, according to a report from IOM, the…
UN Statement on the Renewal of Humanitarian Lifeline to Millions of People in North-West Syria
2021-06-21 17:42
Rome/Geneva/New York – Millions of people are pressed up against the border in an active war zone in north-west Syria and remain in need of humanitarian aid to survive. The UN needs cross-border and cross-line access to reach those most in need.
IVAC [to 26 Jun 2021]
https://www.jhsph.edu/research/centers-and-institutes/ivac/index.html
Updates; Events
Webinar: Vaccines, Primary Care, and Public Health—After COVID, What’s Next?
When: Wednesday, July 7, 2021 from 12:00pm to 1:00pm ET
Join Dr. Tom Frieden, President and CEO of Resolve to Save Lives, for a 60-minute webinar hosted by the International Vaccine Access Center to discuss what roles and strategies vaccines, primary care and public health will play while we prepare for life after COVID-19.
IVI [to 26 Jun 2021]
http://www.ivi.int/
Selected IVI News, Announcements, Events
No new digest content identified.
JEE Alliance [to 26 Jun 2021]
https://www.jeealliance.org/
Selected News and Events
No new digest content identified.
Johns Hopkins Center for Health Security [to 26 Jun 2021]
https://www.centerforhealthsecurity.org/news/center-news/
Center News
25 Stakeholder Groups Call on Congressional Leadership to Invest $30 billion over 4 years to Better Protect Americans from Future Pandemics
June 22, 2021 June 22, 2021 – Today, the Johns Hopkins Center for Health Security joined 24 other stakeholder organizations to submit a letter of support to strongly encourage Congressional leaders to invest $30 billion over 4 years to better protect Americans from future pandemics, as called for in the American Jobs Plan. These funds can create new U.S. jobs in public health, science and technology innovation, and domestic medical countermeasures infrastructure.
Given the devastating consequences of the COVID-19 pandemic, the U.S. should aim to achieve an ambitious but achievable goal of creating a pandemic-free future. Together the 25 stakeholder groups strongly encourage Congress to include this $30 billion investment in a funding vehicle this year…
MSF/Médecins Sans Frontières [to 26 Jun 2021]
http://www.msf.org/
Latest [Selected Announcements
Libya
Ongoing violence against detained migrants forces MSF to suspend Tripoli detention centre activities
Press Release 22 Jun 2021
Cameroon
People in northwest seek healthcare as MSF denied providing medical services
Press Release 22 Jun 2021
Ethiopia Tigray crisis
Tigray violence scatters people across two countries
Crisis Update 22 Jun 2021
National Academy of Medicine – USA [to 26 Jun 2021]
https://nam.edu/programs/
Selected News/Programs
No new digest content identified.
National Vaccine Program Office – U.S. HHS [to 26 Jun 2021]
https://www.hhs.gov/vaccines/about/index.html
Upcoming Meetings/Latest Updates
No new digest content identified.
NIH [to 26 Jun 2021]
http://www.nih.gov/news-events/news-releases
News Releases
International study of rare childhood cancer finds genetic clues, potential for tailored therapy
June 24, 2021 — Rhabdomyosarcoma, or RMS, is a rare cancer that affects the muscles and other soft tissues.
NIH begins study of COVID-19 vaccination during pregnancy and postpartum
June 23, 2021 — Researchers will evaluate antibody responses in vaccinated participants and their infants.
NIH study suggests COVID-19 prevalence far exceeded early pandemic cases
June 22, 2021 — Researchers estimate nearly 17 million undiagnosed cases in the U.S. by mid-July 2020.
UN OCHA Office for the Coordination of Humanitarian Affairs [to 26 Jun 2021]
https://www.unocha.org/
Press Releases
No new digest content identified.
PATH [to 26 Jun 2021]
https://www.path.org/media-center/
Press Releases
No new digest content identified.
Rockefeller Foundation [to 26 Jun 2021]
https://www.rockefellerfoundation.org/
Selected Reports/Press Releases
Jun 23 2021
Press Releases
New Vaccine Equity Cooperative Launches to Advance Covid-19 Vaccine Access and Readiness
Latest Initiative from Collaboration of Racial Health Equity-focused Organizations Includes New Online Resource Hub for Community-Based Workforces
BOSTON, Mass. and OAKLAND, Calif. – The Vaccine Equity Cooperative, a new national collaboration of community health-focused organizations, has launched an online resource hub and information platform to support community-based workforces as they work to increase COVID-19 vaccine access and readiness across the U.S during the most challenging stage of the vaccination effort. As communities of color continue to be disproportionately impacted by vaccine misinformation, mistrust and barriers to access, the website provides curated resources and real-time updates vetted by trusted health care, public health and racial equity experts, as well as opportunities to share content and connect in open forums.
While more than half the adults in the U.S. have been vaccinated, the rate of white people receiving at least one vaccine dose is still 1.4 times higher than that of Black people and 1.2 times higher than that of Hispanic people. People with lingering questions about COVID-19 vaccine safety and access are continuing to turn to trusted local leaders, organizers and health workers in their communities for advice and support about COVID-19 vaccines.
With the country projected to fall short of the Biden administration’s goal of getting 70 percent of American adults at least partially vaccinated by July 4th, supporting local outreach and 1:1 conversations with those facing the most barriers and skepticism will be essential to the success of vaccination efforts, efforts to safely re-open and long-term national public health.
“Community-based and public health workforces have long been underfunded and under-supported, but proved to be absolutely critical to the pandemic response and vaccine rollout,” said Alexandra Quinn, CEO of Health Leads. “The Vaccine Equity Cooperative envisions a world where local workforces have the resources they need – not only to respond to crises, but proactively address ongoing barriers to health. With a goal of enabling racial health equity, our Cooperative will be an ongoing effort to strengthen our public and community health infrastructure through public and private investment, advocacy, and sharing resources and learnings from and with communities across the country.”
Difficulties in accessing accurate information, online registration, language barriers, hard-to-reach vaccination sites and failure of institutions to gain trust are only a few of the factors that have contributed to the disparities in U.S. vaccination rates. While other general population vaccine-related resource hubs exist, the Cooperative’s site offers curated toolkits tailored to community types and drawn from trusted organizations and local leaders.
“Frontline community-based workers and leaders have been the trusted sources and translators of complex health information for generations, but COVID-19 vaccines introduced a host of new data, questions and misinformation,” said Denise Octavia Smith, Executive Director of the National Association of Community Health Workers, a founding partner of the project. “This website helps them navigate readiness and access efforts with community-specific tools and resources from people and organizations that they can trust.”
The Cooperative is encouraging all community-based workforce members to collaborate on the project by giving feedback on published resources, sending questions and requests for information, or sharing new resources and events for publication on the site.
About the Vaccine Equity Cooperative
Visit the resource hub and learn more about the Vaccine Equity Cooperative at www.vaccineequitycooperative.org.
The Vaccine Equity Cooperative is a national collaboration of organizations committed to racial health equity, including Health Leads, CONVINCE USA, National Association of Community Health Workers, Native Ways Federation and Partners In Health. The Cooperative works to increase access to trusted health resources, expand investment in community-based organizations, and strengthen policy in support of community-based and public health workforces. The Cooperative is supported by The Rockefeller Foundation, The JPB Foundation, The W.K. Kellogg Foundation, and The Kresge Foundation. For more information and the Cooperative’s resource hub, visit www.vaccineequitycooperative.org.
Sabin Vaccine Institute [to 26 Jun 2021]
http://www.sabin.org/updates/pressreleases
Statements and Press Releases
No new digest content identified.
UNAIDS [to 26 Jun 2021]
http://www.unaids.org/en
Selected Press Releases/Reports/Statements
25 June 2021
Training on data on the location and size estimates of key populations in western and central Africa
21 June 2021
Small steps towards a big goal
21 June 2021
The importance of young people’s sexual and reproductive health and rights to the global HIV response
UNHCR Office of the United Nations High Commissioner for Refugees [to 26 Jun 2021]
http://www.unhcr.org/en-us/media-centre.htmlS
Selected News Releases, Announcements
UNHCR calls on states to remove barriers to access to COVID-19 vaccines for refugees
24 June 2021
[See COVID above for detail]
UN agencies welcome relocation of 4,000 asylum seekers and refugees from Greece
IOM, UNHCR and UNICEF jointly encourage further steps towards a predictable, systematic European relocation mechanism.
24 June 2021
UN High Commissioner for Refugees praises Latin America for its commitment to the inclusion of all those in need of protection
23 June 2021
Quito, Ecuador – At the end of a week-long visit to three Latin American countries, UN High Commissioner for Refugees Filippo Grandi applauded the region for its solidarity and commitment to protect refugees, asylum seekers and other displaced people.
“Latin American countries are facing an unprecedented level of displacement,” said Grandi. “Yet, they have stepped up to the challenge by showing unique generosity and dedication to find dignified solutions for those forced to flee.”
The Americas region is home to 20 per cent of the 82.4 million people forcibly displaced globally, including the second-largest external displacement crisis in the world – the 5.6 million refugees and migrants from Venezuela. As Latin America struggles to contain the COVID-19 pandemic, several countries have initiated large-scale regularization programmes to realize the full potential of displaced people and the contribution they can make to their communities.
“Inclusion is one of the most practical and concrete forms of protection. It helps children to get an education, people to receive the medical treatment they need, prevents exploitation and abuse and supports people to acquire the dignity of self-sustaining work,” explained Grandi. “Inclusion is the new protection.”…
UNICEF [to 26 Jun 2021]
https://www.unicef.org/media/press-releases
Selected Press Releases, Statements
News note 06/25/2021
Geneva Palais briefing note on the worsening situation in Sub Saharan Africa as a result of secondary impacts of COVID-19
This is a summary of what was said by UNICEF spokesperson James Elder – to whom quoted text may be attributed – at today’s press briefing at the Palais des Nations in Geneva
GENEVA, 25 June 2021 – “Sub-Saharan Africa is in the throes of a deadly uptake in COVID-19. At the present rate of infections, the current surge will exceed the previous one within weeks. As more contagious variants spread, vaccines continue to be perilously slow in reaching Africa, and hospitals are pushed beyond capacity.
“Amid it all, the impacts on children continue to be devastating. So as to provide a very quick regional snapshot:
:: In Uganda there has been a 2,800 per cent increase in new COVID19 cases between March and June 2021. The availability of oxygen in Uganda become a life or death situation.
:: Namibia, last week, had the highest death rate in Africa. Hospitals are full and there are not enough oxygen tanks. According to the Ministry of Health, Namibia is experiencing over 1,000 new COVID19 cases each day and 30 deaths. That is a high death rate for a country of 2.5 million people.
:: In the Democratic Republic of the Congo, it’s an equally daunting picture – with low vaccination rates and poor health facilities.
:: And in South Africa a third wave is threatening to be even worse than the previous two, stretching an already strained healthcare system. So far, only 2.5 million people have received at least one vaccination, from a population of around 57 million. And yet that’s one of the higher vaccination rates on the continent.
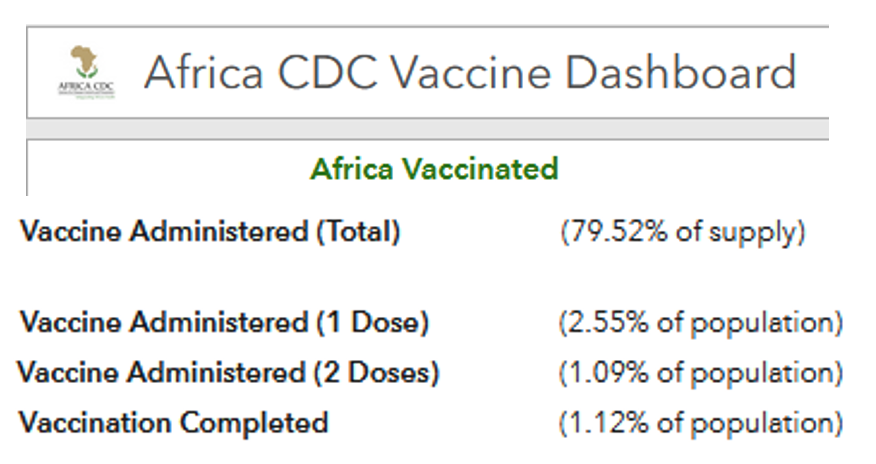
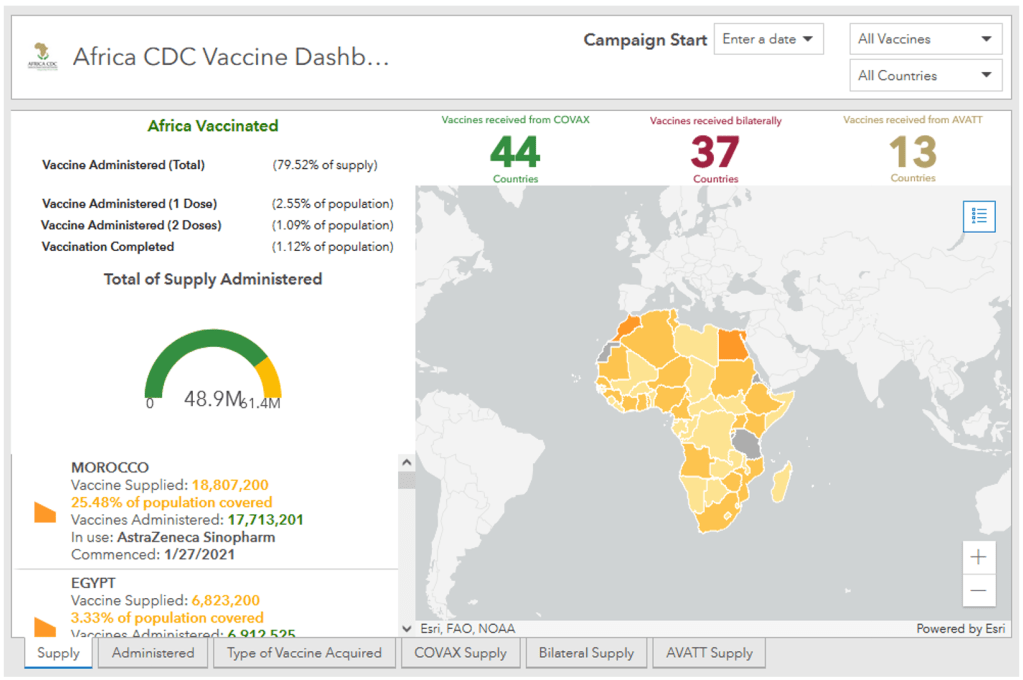
:: Indeed, if we look at the situation across the world, there have been about 2.7 billion doses administered. Of these, around just 1.5% have been administered on the continent.
“What does all this look like for a child in Sub Saharan Africa?
“It looks like the loss of parents, and grandparents who care for so many children; It looks like less education and more abuse. COVID-19 has meant a devastating blow to education. For instance, UNICEF estimates 9 million children in Eastern and Southern Africa never returned to class as schools started opening; And now schools that re-opened are starting to close again; “it looks – and feels – like more anxiety and stress for children, as isolation, confinement, and loss of income take their toll…
Unitaid [to 26 Jun 2021]
https://unitaid.org/
Featured News
Unitaid’s Executive Board meets to discuss strategic direction in an evolving global health landscape at 38th meeting
Geneva, 18 June 2021 – The Executive Board recognized Unitaid’s leadership and agility in responding to COVID-19 and discussed new challenges and opportunities facing the organization during its 38th meeting, held virtually from 16th to 18th June.
Unitaid’s board reaffirmed its commitment to its core work and explored lessons learned from the pandemic response and ways its innovative model and expertise could lend itself to additional contexts.
As Unitaid continues its strategy development process for 2022-2026, key among the considerations discussed was how to define ambitions and priorities in a way that allows enough flexibility to ensure success in an increasingly challenging global health environment…
Vaccination Acceptance & Demand Initiative [Sabin) [to 26 Jun 2021]
https://www.vaccineacceptance.org/
Announcements
No new digest content identified.
Vaccine Confidence Project [to 26 Jun 2021]
http://www.vaccineconfidence.org/
News, Research and Reports
Coronavirus global impact
Launched April 2, 2020 and recurring every 3 days, Premise Data is utilizing its global network of Contributors to assess economic, social, and health sentiment surrounding the coronavirus (COVID-19).
Vaccine Education Center – Children’s Hospital of Philadelphia [to 26 Jun 2021]
http://www.chop.edu/centers-programs/vaccine-education-center
News
No new digest content identified.
Wellcome Trust [to 26 Jun 2021]
https://wellcome.ac.uk/news
News and reports
Explainer
What treatments are working for Covid-19?
From existing antivirals to new antibody therapies – researchers are working tirelessly to find the best drugs to treat Covid-19.
23 June 2021
The Wistar Institute [to 26 Jun 2021]
https://www.wistar.org/news/press-releases
Press Releases
No new digest content identified.
WFPHA: World Federation of Public Health Associations [to 26 Jun 2021]
https://www.wfpha.org/
Latest News
No new digest content identified.
World Bank [to 26 Jun 2021]
http://www.worldbank.org/en/news/all
Selected News, Announcements
US$30m Boost for PNG’s COVID-19 Response
World Bank announces additional support for COVID-19 response and vaccine rollout PORT MORESBY, June 25, 2021 – The World Bank has approved an additional US$30 million (approximately PGK70m) in funding…
Date: June 25, 2021 Type: Press Release
Additional Financing Support by the Green Climate Fund to Help Strengthen Climate Resilience in Central Asia
WASHINGTON, June 24, 2021 — The World Bank, as an accredited entity for the Green Climate Fund (GCF), approved today additional financing totaling $19 million for the Climate Adaptation and Mitigation…
Date: June 24, 2021 Type: Press Release
New $200 Million World Bank Project to Support COVID-19 Relief, Build Resilience Against Future Economic Shocks in Cambodia
WASHINGTON, June 24, 2021 – Cambodia’s efforts to combat the ongoing COVID-19 pandemic and begin its journey toward economic recovery were given a boost today by World Bank’s Board of Executive Directors…
Date: June 24, 2021 Type: Press Release
World Bank Approves US$100 Million for Barbados’ COVID-19 Response and Recovery
WASHINGTON, June 24, 2021 – The World Bank Board of Executive Directors approved today a US$100 million COVID-19 Response and Recovery Development Policy Loan for Barbados. The operation will support the…
Date: June 24, 2021 Type: Press Release
New Grant to Sustain Afghanistan’s Reforms toward COVID-19 Recovery
Washington, June 24, 2021— The World Bank Board of Executive Directors today approved a $132 million grant from the International Development Association (IDA) to help Afghanistan continue implementing…
Date: June 24, 2021 Type: Press Release
Remarks by World Bank Group President David R. Malpass: Avoiding a Lost Decade in Latin America and the Caribbean (LAC)
Thank you, Maria, the Andean American Associations, and all participants throughout the Latin American and Caribbean regions. I also want to thank Juana Caicedo, President of the Ecuadorean American Association…
Date: June 23, 2021 Type: Speeches and Transcripts
US$63.75 Million Additional Financing to Support COVID-19 Vaccination in Jordan
Washington DC, June 23, 2021 – The World Bank approved on June 16, 2021, US$63.75 million in additional financing for the ongoing Jordan COVID-19 Emergency Response project to support Jordan’s efforts…
Date: June 23, 2021 Type: Press Release
World Bank and African Union Team Up to Support Rapid Vaccination for Up to 400 million People in Africa
Working with countries and partners across Africa to quickly expand equitable access to vaccines WASHINGTON, June 21, 2021— The African Finance Ministers and the World Bank Group met today to fast…
Date: June 21, 2021 Type: Press Release
World Customs Organization – WCO [to 26 Jun 2021]
http://www.wcoomd.org/
Latest News – Selected Items
26 June 2021
WCO Council renews support for the Secretariat in preparation for a post-pandemic world
Heads of Delegation of some 130 World Customs Organization (WCO) Member Customs administrations took part in the virtual meeting of the 138th Session of the WCO Council, held from 24 to 26 June 2021. As the world is bracing for recovery with a glimmer of optimism emerging from the global effort regarding vaccinations, this session focused on Capacity Building, Rules of Origin, Valuation, Nomenclature and Classification, Compliance and Trade Facilitation as well as budgetary and financial matters…
World Organisation for Animal Health (OIE) [to 26 Jun 2021]
https://www.oie.int/en/for-the-media/press-releases/2021/
Press Releases
No new digest content identified.
::::::
ARM [Alliance for Regenerative Medicine] [to 26 Jun 2021]
Press Releases – Alliance for Regenerative Medicine (alliancerm.org)
Selected Press Releases
No new digest content identified.
BIO [to 26 Jun 2021]
https://www.bio.org/press-releases
Press Releases
No new digest content identified.
DCVMN – Developing Country Vaccine Manufacturers Network [to 26 Jun 2021]
http://www.dcvmn.org/
News; Upcoming events
No new digest content identified.
ICBA – International Council of Biotechnology Associations [to 26 Jun 2021]
https://internationalbiotech.org/news/
News
No new digest content identified.
IFPMA [to 26 Jun 2021]
http://www.ifpma.org/resources/news-releases/
Selected Press Releases, Statements, Publications
New Non-State Actors Alliance calls on behalf of patients, industry for urgent action to set
22 June – Paris/Geneva/London – Ministers of Health from four African countries (Algeria, Democratic Republic of Congo, Egypt and Cabo Verde), representatives from international organizations, patient groups and the pharmaceutical industry reiterated today at a high-level roundtable event the urgency of establishing a regulatory authority across Africa, especially in the context of the COVID-19 pandemic. The current fragmented regulatory systems across the continent are making it difficult to mount an appropriate response.
Ministers of Health and roundtable participants outlined the first areas of work for the AMA and agreed that it has the unique opportunity to become one of the most efficient and modern regulatory systems in the world. Once established, it will perform a vital task in overseeing rapid and effective market authorisation of safe, quality, effective and accessible vaccines, medicines, and health devices to control and treat disease across Africa to robust regulatory standards. Crucially, it will foster reliance and regulatory harmonization across the continent. In practice, this means that national regulatory authorities will be able to build on the work done by counterparts in other countries, significantly cutting down the time it takes for medicines, vaccines or diagnostics to reach the market. For many countries, the AMA also holds the promise of driving industrial and economic growth, through encouraging the development of local pharmaceutical industry and the establishment of centres of excellence for research across the continent.
But while commitment to the AMA mission and vision is strong, it has not translated into concrete actions. A new cross-stakeholder alliance announced at the roundtable, the African Medicines Agency Treaty Alliance (AMATA), will seek to push for rapid ratification of the Treaty, as well as meaningful engagement with patients, industry and other relevant parties once the Agency becomes operational…
Biopharmaceutical CEO Brussel meeting with EU leaders focuses on equitable access to tackle COVID-19, fostering
Brussels, 22 June 2021 – The Biopharmaceutical CEO Roundtable (BCR), which represents the world’s leading biopharmaceutical companies, held their first face-to-face meeting since December 2019 in Brussels on Monday 21 and Tuesday 22 June, to discuss global health challenges and public health policies impacting biomedical innovation.
The fourteen biopharmaceutical industry CEOs who attended in person represent a global industry which is heavily invested in Europe, as key players in the research and development ecosystem, local economies and with health care systems. Their programme…covered a range of topics, including the development and scaling up of COVID-19 vaccines and how to foster an environment that invests in innovation. The CEOs explained the incredible innovations coming down the pipeline for patients, such as combination therapies, advanced therapies and Artificial Intelligence guided therapy.
The multifaceted root cause of unavailability and delay to innovative medicines were discussed, and the need to find solutions to reduce the time before patients have access to innovative medicines. The group tabled a number of proposals including the establishment of an industry portal targeting European access hurdles reflecting the shared aspiration of companies to file pricing and reimbursement applications within 2 years from the granting of the marketing authorization. The CEO delegation made concrete proposals to ensure equity of access and solidarity across EU member states, by means of equity-based tiered pricing, which would require an intergovernmental framework for it to be implementable and effective. There was clearly a shared commitment to ensure safe and efficacious innovative products reach the market and patients, enabled by a first-class regulatory system. The EU’s regulatory system, which stood up well to the pandemic challenges, could remove the bottlenecks and catch up with regulatory systems in other parts of the world in terms of speed and flexibility of assessment by rationalising the decision making (expertise based) and reducing interfaces…
PhRMA [to 26 Jun 2021]
http://www.phrma.org/
Latest News
More than 800 medicines are in development for diseases that disproportionately affect racial and ethnic communities
June 22, 2021
PhRMA released a new report exploring the 829 medicines in development that aim to address the diseases and conditions that affect racial and ethnic communities at a higher rate.
Blog Post