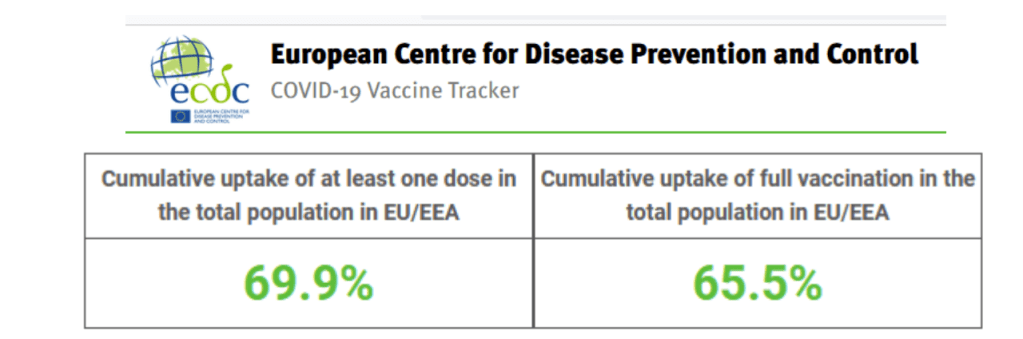
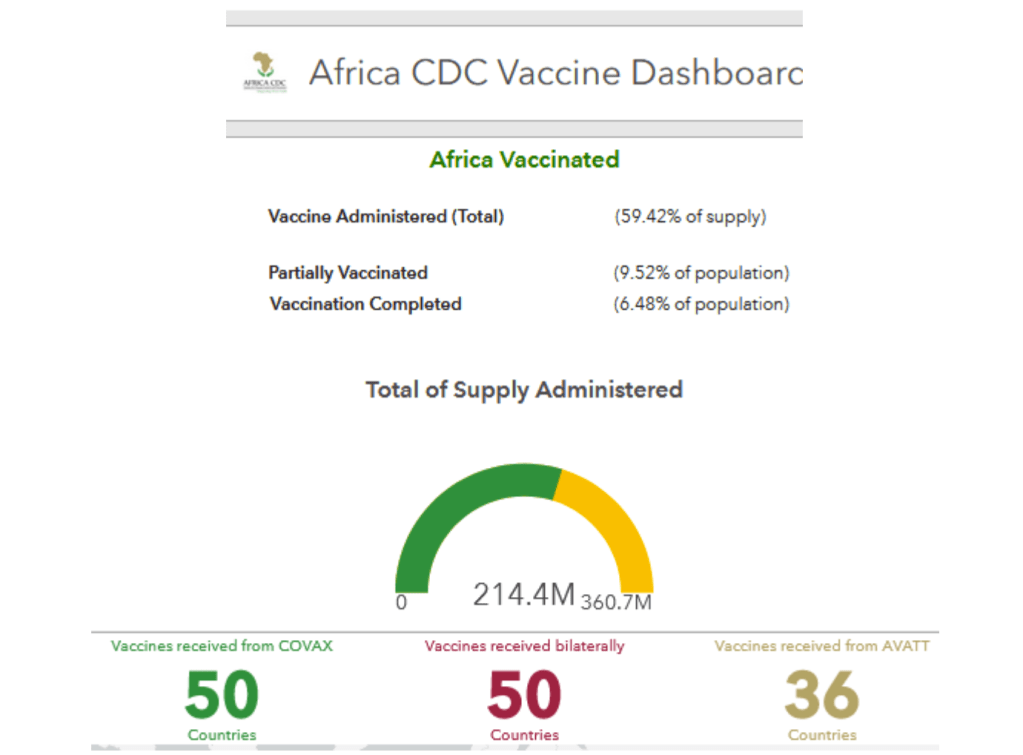
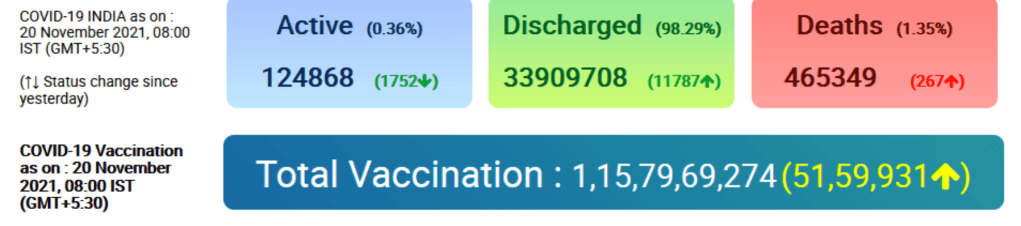
Vaccines and Global Health: The Week in Review is a weekly digest summarizing news, events, announcements, peer-reviewed articles and research in the global vaccine ethics and policy space. Content is aggregated from key governmental, NGO, international organization and industry sources, key peer-reviewed journals, and other media channels. This summary proceeds from the broad base of themes and issues monitored by the Center for Vaccine Ethics & Policy in its work: it is not intended to be exhaustive in its coverage. You are viewing the blog version of our weekly digest, typically comprised of between 30 and 40 posts below all dated with the current issue date
.– Request an Email Summary: Vaccines and Global Health : The Week in Review is published as a single email summary, scheduled for release each Saturday evening before midnight (EDT in the U.S.). If you would like to receive the email version, please send your request to david.r.curry@centerforvaccineethicsandpolicy.org.
– pdf version: A pdf of the current issue is available here:
– blog edition: comprised of the approx. 35+ entries posted below.
– Twitter: Readers can also follow developments on twitter: @vaxethicspolicy.
.
– Links: We endeavor to test each link as we incorporate it into any post, but recognize that some links may become “stale” as publications and websites reorganize content over time. We apologize in advance for any links that may not be operative. We believe the contextual information in a given post should allow retrieval, but please contact us as above for assistance if necessary.
Support this knowledge-sharing service: Your financial support helps us cover our costs and to address a current shortfall in our annual operating budget. Click here to donate and thank you in advance for your contribution.
.
David R. Curry, MS
Executive Director
Center for Vaccine Ethics and Policy