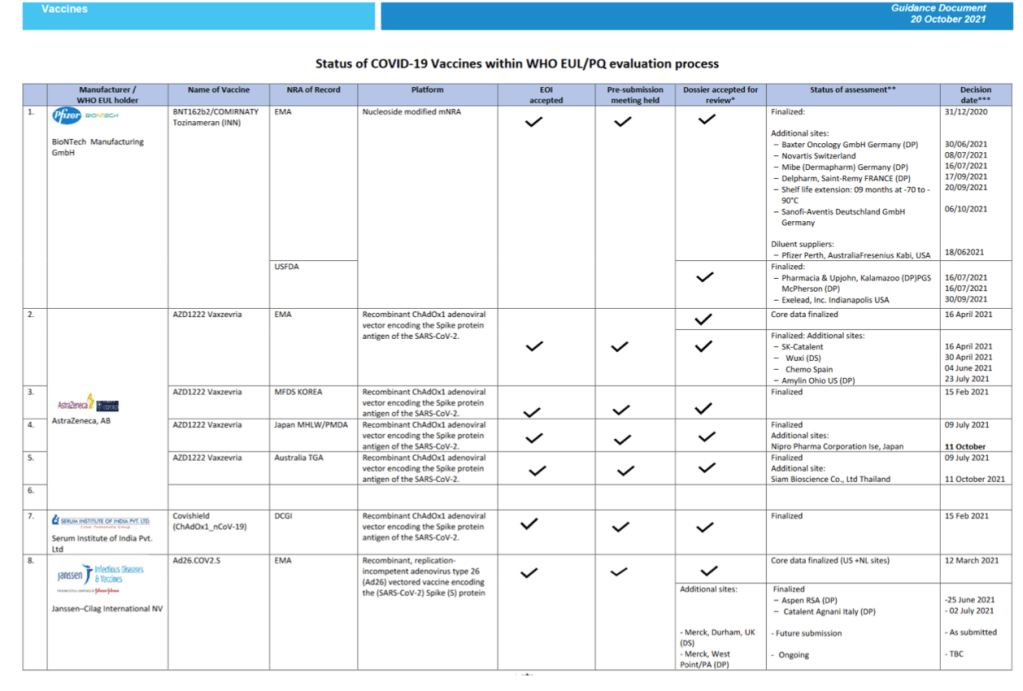
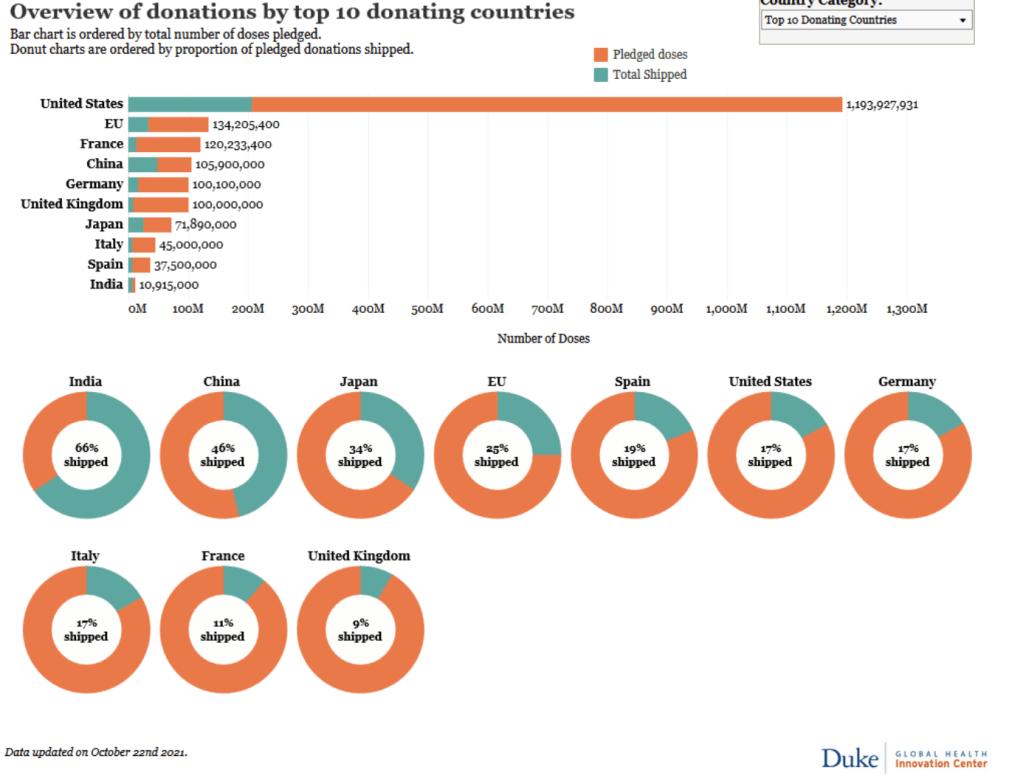
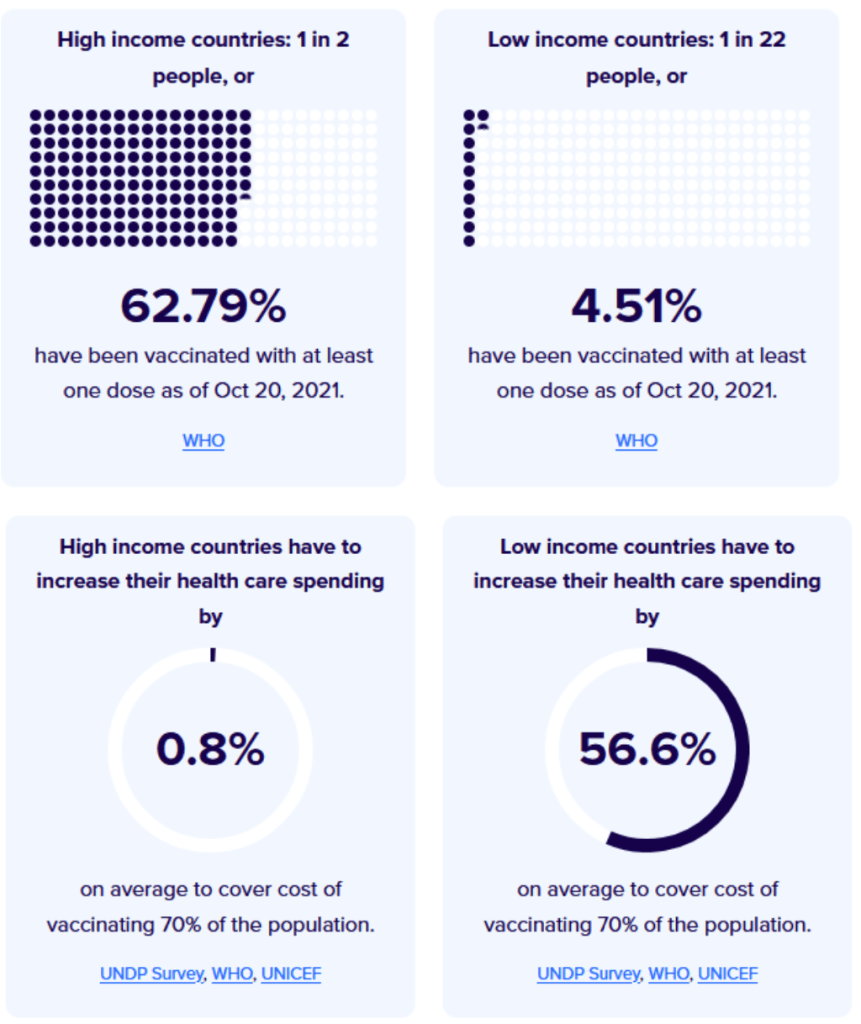
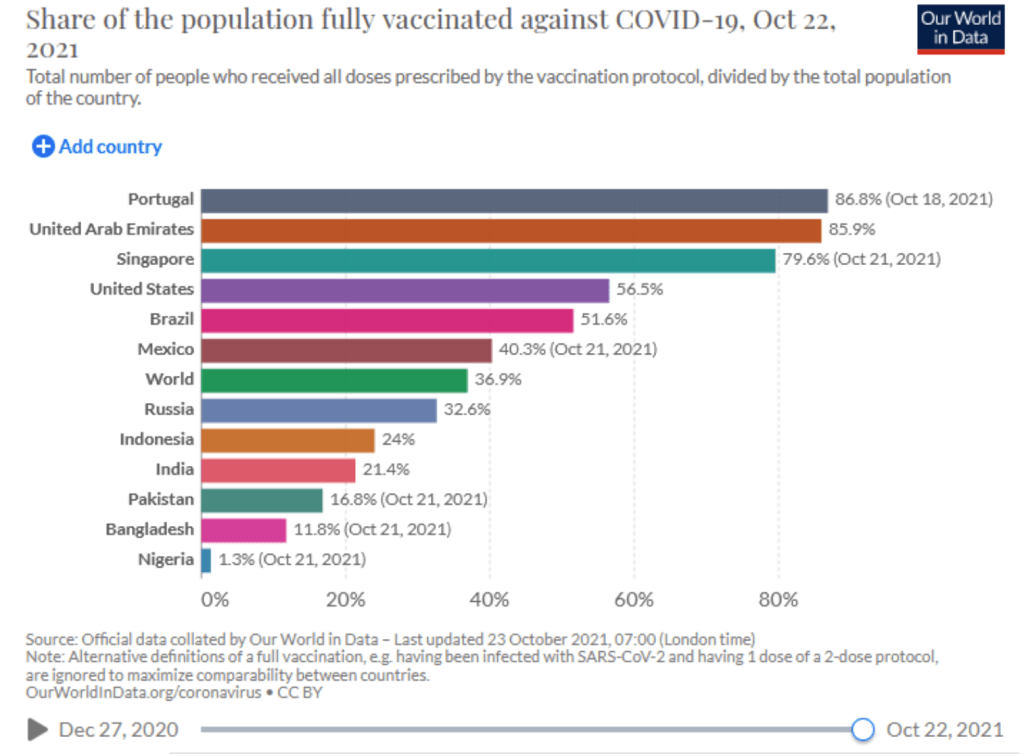
::::::
Organization Announcements
Editor’s Note:
Careful readers will note that the number and range of organizations now monitored in our Announcements section below has grown as the impacts of the pandemic have spread across global economies, supply chains and programmatic activity of multilateral agencies and INGOs.
Paul G. Allen Frontiers Group [to 23 Oct 2021]
https://alleninstitute.org/what-we-do/frontiers-group/new s-press/
News
No new digest content identified.
BARDA – U.S. Department of HHS [to 23 Oct 2021]
https://www.phe.gov/about/barda/Pages/default.aspx
News
No new digest content identified.
BMGF – Gates Foundation [to 23 Oct 2021]
https://www.gatesfoundation.org/ideas/media-center
Press Releases and Statements
Bill & Melinda Gates Foundation Commits up to $120 Million to Accelerate Access to COVID-19 Drug for Lower-Income Countries, Calls on Other Donors to Mobilize Resources
Financial commitment aims to secure dedicated, low-cost supply of molnupiravir, if the drug is authorized by regulators; builds on long-term efforts to increase access to tests, treatments, vaccines
Oct 19, 2021
… This commitment builds on the foundation’s ongoing efforts, including $1.9 billion in funding, since the start of the pandemic to increase access to COVID-19 vaccines, treatments, and tests by supporting R&D, regulatory work, at-risk manufacturing, and product delivery…
Bill & Melinda Gates Medical Research Institute [to 23 Oct 2021]
https://www.gatesmri.org/
The Bill & Melinda Gates Medical Research Institute is a non-profit biotech organization. Our mission is to develop products to fight malaria, tuberculosis, and diarrheal diseases—three major causes of mortality, poverty, and inequality in developing countries. The world has unprecedented scientific tools at its disposal; now is the time to use them to save the lives of the world’s poorest people
No new digest content identified.
CARB-X [to 23 Oct 2021]
https://carb-x.org/
News
No new digest content identified.
Center for Vaccine Ethics and Policy – GE2P2 Global Foundation [to 23 Oct 2021]
https://centerforvaccineethicsandpolicy.net/
News/Analysis/Statements
:: Past weekly editions and posting of all segments of Vaccines and Global Health: The Week in Review are available here.
:: [NEW] Informed Consent: A Monthly Review – October 2021 is now posted here
CEPI – Coalition for Epidemic Preparedness Innovations [to 23 Oct 2021]
http://cepi.net/
Latest News
No new digest content identified.
DARPA – Defense Advanced Research Projects Agency [to 23 Oct 2021
https://www.darpa.mil/news
News
No new digest content identified.
Duke Global Health Innovation Center [to 23 Oct 2021]
https://dukeghic.org/
Our Blog
No new digest content identified.
EDCTP [to 23 Oct 2021]
http://www.edctp.org/
The European & Developing Countries Clinical Trials Partnership (EDCTP) aims to accelerate the development of new or improved drugs, vaccines, microbicides and diagnostics against HIV/AIDS, tuberculosis and malaria as well as other poverty-related and neglected infectious diseases in sub-Saharan Africa, with a focus on phase II and III clinical trials
News
18 October 2021
The Tenth EDCTP Forum has started
Join the Forum proceedings (registration only)
Emory Vaccine Center [to 23 Oct 2021]
http://www.vaccines.emory.edu/
Vaccine Center News
No new digest content identified.
European Vaccine Initiative [to 23 Oct 2021]
http://www.euvaccine.eu/
Latest News
No new digest content identified.
Fondation Merieux [to 23 Oct 2021]
http://www.fondation-merieux.org/
News, Events
No new digest content identified.
Gavi [to 23 Oct 2021]
https://www.gavi.org/
News releases
18 October 2021
First Portuguese COVAX doses reach Senegal
GHIT Fund [to 23 Oct 2021]
https://www.ghitfund.org/newsroom/press
GHIT was set up in 212 with the aim of developing new tools to tackle infectious diseases that
No new digest content identified.
Global Fund [to 23 Oct 2021]
https://www.theglobalfund.org/en/news/
News & Stories
No new digest content identified.
Global Research Collaboration for Infectious Disease Preparedness [GloPID-R] [to 23 Oct 2021]
https://www.glopid-r.org/news/
News
No new digest content identified.
Hilleman Laboratories [to 23 Oct 2021]
http://www.hillemanlabs.org/
Website reports “under maintenance” at inquiry
Human Vaccines Project [to 23 Oct 2021]
http://www.humanvaccinesproject.org/
News
Press Release
Oct 22, 2021
Lawrence Livermore National Laboratory joins Human Vaccines Project
IAVI [to 23 Oct 2021]
https://www.iavi.org/newsroom
Latest News
October 20, 2021
TB survivors from G20 countries call upon their leaders to invest $1 billion per year to develop new TB vaccines
The call is endorsed by a global coalition advocating for a fully funded and resourced TB vaccine pipeline by 2023
October 20, 2021
In a direct address to their Heads of State and Government, a group of tuberculosis (TB) survivors from the G20 nations sent a letter to their political leaders as they prepare to gather at the G20 Summit in Rome, calling upon them to uphold their promise made at the United Nations High-Level Meeting (UN HLM) on TB in 2018 to develop new vaccines against TB. Written by a group of TB survivors and signed by TB survivors from each of the G20 countries and over 55 organizations around the world, the letter asks G20 leaders to secure an annual investment of at least US$1 billion in TB vaccine research so that new TB vaccines are possible as early as 2025.
The letter is endorsed by the TB Vaccine Advocacy Roadmap (TB Vax ARM), a new global coalition of TB advocates and allied organizations spearheaded by IAVI, Results UK, and TAG. The coalition has come together to accelerate TB vaccine development by ensuring a fully funded and resourced TB vaccine pipeline by 2023, a requirement for delivering new TB vaccines in time to end the TB epidemic by 2030, the goal set at the UN HLM in 2018.
In the letter, TB survivors remind their leaders that even in the face of COVID-19, which killed 1.7 million people in 2020, TB remained the leading infectious cause of death in much of the world: “TB is a disease of inequity — over 90% of people who fall sick with TB live in developing and emerging economies. New TB vaccines could help protect us from the suffering we have experienced and save the precious lives and livelihoods of millions around the world from the leading infectious killer before COVID-19. We call on G20 countries to commit the resources needed to develop life-saving vaccines so that we can truly end TB during this decade,’’ said Rhea Lobo, a TB survivor from India who helped lead this initiative…
October 18, 2021
Vaccines Security in Africa – Which Way Next?
…“Vaccines Security in Africa – Which Way Next?” brought together researchers, policymakers, pharmaceutical/biotech companies, civil society organizations, and the media to discuss and advocate for pathways toward a sustainable vaccine development and manufacturing ecosystem backed with sustainable financing, strategic partnerships, innovative financing approaches, and domestic resource mobilization. Forum participants also highlighted opportunities to accelerate vaccine development and manufacturing in Africa. Speakers included representatives of Africa Centres for Disease Control and Prevention (CDC), AVMI, IAVI, WHO, and WHO Regional office for Africa (WHO/AFRO).
Keynote speaker Dr. Lindiwe Makubalo, assistant regional director, WHO/AFRO, stated that now is not the time to ask for political will, but rather political action, leadership, and practical support aimed at accelerating sustainable access to vaccines on the continent. She noted that COVID-19 presents new opportunities for action in domestic vaccine manufacturing and reiterated WHO’s commitment to partnering with governments, policymakers, and regulators to support a sustainable market for locally produced vaccines….
International Coalition of Medicines Regulatory Authorities [ICMRA]
http://www.icmra.info/drupal/en/news
Selected Statements, Press Releases, Research
No new digest content identified.
ICRC [to 23 Oct 2021]
https://www.icrc.org/en/whats-new
Selected News Releases, Statements, Reports
Statement on behalf of the 160 signatories to the Climate and Environment Charter for Humanitarian Organizations to the 26th UNFCCC COP
“Today’s climate and environmental crises threaten the survival of humanity. All dimensions of our lives are affected, from our physical and mental health to our food, water and economic security.
20-10-2021 | Statement
IFFIm
http://www.iffim.org/
Press Releases/Announcements
No new digest content identified.
IFRC [to 23 Oct 2021]
http://media.ifrc.org/ifrc/news/press-releases/
Selected Press Releases, Announcements
Red Cross rushes relief as severe floods and landslides hit Nepal, India
21/10/2021 | Press release
Institut Pasteur [to 23 Oct 2021]
https://www.pasteur.fr/en/press-area
Press Documents
No new digest content identified.
IOM / International Organization for Migration [to 23 Oct 2021]
http://www.iom.int/press-room/press-releases
News – Selected
News 19 Oct 2021
Djibouti Rolls Out COVID-19 Vaccinations for Migrants
Djibouti – Migrants in Djibouti are being vaccinated against COVID-19 for the first time, as the International Organization for Migration (IOM) works with the Government to support the national immunization effort.
Since the start of the COVID-19 vaccine roll-out globally, IOM has been advocating for the inclusion of migrants and Djibouti is one of the first countries in the region to initiate a campaign for them. Around 70 migrants have received jabs since the vaccination drive began on 12 October and it will continue till at least the end of the year…
ISC / International Science Council [to 23 Oct 2021]
https://council.science/current/
ISC is a non-governmental organization with a unique global membership that brings together 40 international scientific Unions and Associations and over 140 national and regional scientific organizations including Academies and Research Councils.
News
Freedom and responsibility in the 21st century: a contemporary perspective on the free and responsible practice of science
CFRS Draft Discussion Paper
September 2021 ;; 41 pages
The Paper is intended for a broad readership, including researchers, research managers, policymakers, science diplomats, and those in the private sector. As such, we invite you to identify a range of readers affiliated with your organization who can review the content of the draft Paper from these diverse perspectives.
6. Conclusion
This Discussion Paper concludes on an optimistic note. Science is a unique human activity that, over time, has given us deep knowledge of ourselves and our place in the universe. Broadly defined, the sciences, including technology, the social sciences and humanities, have played vital roles in the human story in the 21st century. Researchers are key members of contemporary society. Their contribution to human wellbeing and to planetary health is maximized when they are allowed appropriate freedoms to meet their individual and collective responsibilities. The international scientific community, governments, the public, and private research institutions should each have a clear sense of their freedoms and responsibilities, and a clear strategy to achieve the free and responsible practice of scientific research.
Draft Report on the ISC Strategy in the Intergovernmental System
ISC/GA-2/DOC.17.1
October 2021 :: 13 pages
In March 2021, the ISC Chief Executive Officer Heide Hackmann invited Julia Marton-Lefèvre to chair a Steering Group to lead the development of a strategy for the ISC to engage with the intergovernmental system in order to enhance the impact of the Council and strengthen the voice of science in global policy processes…
IVAC [to 23 Oct 2021]
https://www.jhsph.edu/research/centers-and-institutes/ivac/index.html
Updates; Events
No new digest content identified.
IVI [to 23 Oct 2021]
http://www.ivi.int/
IVI News & Announcements
No new digest content identified.
Johns Hopkins Center for Health Security [to 23 Oct 2021]
https://www.centerforhealthsecurity.org/news/center-news/
Center News
New Local Research Report Released on Improving COVID-19 Vaccination Equity for Latino Populations in Baltimore City
October 20, 2021
Johns Hopkins Center for Health Security Contributing Scholar Tener Goodwin Veenema Elected to National Academy of Medicine
October 18, 2021
New Report: Striving Toward Health Equity in COVID-19 – The Role of Pharmacies in a National Response
October 18, 2021
MSF/Médecins Sans Frontières [to 23 Oct 2021]
http://www.msf.org/
Latest [Selected Announcements]
Tuberculosis
Clinical trial results offer hope to DR-TB patients with short, effective treatment
Press Release 20 Oct 2021
Tuberculosis
EndTB clinical trial for multidrug-resistant TB completes enrolment
Press Release 18 Oct 2021
National Academy of Medicine – USA [to 23 Oct 2021]
https://nam.edu/programs/
Selected News/Programs/Events
National Academy of Medicine Elects 100 New Members
October 18, 2021
The National Academy of Medicine (NAM) today announced the election of 90 regular members and 10 international members during its annual meeting. Election to the Academy is considered one of the highest honors in the fields of health and medicine and recognizes individuals who have demonstrated outstanding professional achievement and commitment to service. “It is […]
[Excerpt]
Newly elected regular members of the National Academy of Medicine and their election citations include:
Kathrin U. Jansen, PhD, senior vice president and head of vaccine research and development, Pfizer Inc. For leading the teams that produced three revolutionary vaccines: Gardasil, targeting human papillomavirus; Prevnar 13, targeting 13 strains of pneumococcus; and the Pfizer/BioNTech SARS-Covid-2 mRNA vaccine.
Nancy Messonnier, MD, executive director, pandemic prevention and health systems, Skoll Foundation. For her efforts in tackling the COVID-19 pandemic and building a global preparedness and response system to prevent future pandemics.
Rochelle Paula Walensky, MD, MPH, director, Centers for Disease Control and Prevention. For her work that motivated changes to HIV and COVID-19 guidelines, influenced public health practice, and provided rigorous evidence for decisions by the U.S. Congress, the World Health Organization, and Joint United Nations Programme on HIV/AIDS.
National Academy of Medicine Announces Creation of David and Beatrix Hamburg Award for Advances in Biomedical Research and Clinical Medicine
October 17, 2021
The National Academy of Medicine (NAM) has announced the creation of a new award — the David and Beatrix Hamburg Award for Advances in Biomedical Research and Clinical Medicine — to recognize creative and accomplished biomedical scientists who are advancing health and the human condition around the world.
National Academy of Sciences – USA [to 23 Oct 2021]
http://www.nasonline.org/news-and-multimedia/
News
No new digest content identified.
National Vaccine Program Office – U.S. HHS [to 23 Oct 2021]
https://www.hhs.gov/vaccines/about/index.html
Upcoming Meetings/Latest Updates
No new digest content identified.
NIH [to 23 Oct 2021]
http://www.nih.gov/news-events/news-releases
News Releases
COVID vaccine booster increases antibody responses, is protective in rhesus macaques
October 21, 2021 — Different mRNA-1273 boosters are equally protective against variants.
Interferon does not improve outcomes for hospitalized adults with COVID-19
October 18, 2021 — Results of NIH clinical trial published today.
PATH [to 23 Oct 2021]
https://www.path.org/media-center/
Press Releases
New HPV vaccine from Innovax receives WHO prequalification
This important milestone will provide countries with an additional option for HPV vaccination at an affordable price and will contribute to sustainable supply of HPV vaccine—allowing more girls to be reached with HPV vaccines.
October 19, 2021 b
Cecolin®, a vaccine against human papillomavirus (HPV), has received prequalification by the World Health Organization (WHO). Cecolin is manufactured by Xiamen Innovax Biotech CO., LTD. (Innovax), a wholly owned subsidiary of Beijing Wantai Biological Pharmaceutical Co., LTD. (Wantai), and is designed to protect against HPV types 16 and 18, the most common virus types that lead to cervical cancer. Countries facing barriers to national introduction or expanding their HPV vaccine program to the full cohort due to price or supply constraints will now have another option for affordable, sustainable access….
Prior to Cecolin’s licensure in China, PATH provided Innovax with technical assistance for the WHO prequalification process. Activities included improving the quality management system, establishing a post-marketing pharmacovigilance system, and collecting information on regulatory requirements for registration of vaccines in Gavi countries…
Prior to Cecolin’s licensure in China, PATH provided Innovax with technical assistance for the WHO prequalification process. Activities included improving the quality management system, establishing a post-marketing pharmacovigilance system, and collecting information on regulatory requirements for registration of vaccines in Gavi countries.
Sabin Vaccine Institute [to 23 Oct 2021]
http://www.sabin.org/updates/pressreleases
Statements and Press Releases
Sabin Vaccine Institute Receives Additional $34.5 Million from BARDA for Further Development of Ebola Sudan and Marburg Vaccines
Thursday, October 21, 2021
The Sabin Vaccine Institute (Sabin) announced that the Biomedical Advanced Research and Development Authority (BARDA), part of the Office of the Assistant Secretary for Preparedness and Response within the U.S. Department of Health and Human Services, has exercised the third contract option, valued at $34.5 million, under the 2019 contract to advance the development of vaccines against Ebola Sudan and Marburg viruses through Phase 2 clinical trials…
UNAIDS [to 23 Oct 2021]
http://www.unaids.org/en
Selected Press Releases/Reports/Statements
21 October 2021
A Dose of Reality: How rich countries and pharmaceutical corporations are breaking their vaccine promises
19 October 2021
Strengthening the response of health systems to pandemics in the Commonwealth of Independent States
18 October 2021
“Realizing the right to reproductive health and the future starts with sexual education”
18 October 2021
Capitalizing on experiences to improve HIV care for key populations in western Africa
18 October 2021
Upper-middle-income countries pay more for HIV medicines, but price reductions can be achieved
UNHCR Office of the United Nations High Commissioner for Refugees [to 23 Oct 2021]
http://www.unhcr.org/en-us/media-centre.htmlS
Selected News Releases, Announcements
No new digest content identified.
UNICEF [to 23 Oct 2021]
https://www.unicef.org/media/press-releases
Press Releases, News Notes, Statements [Selected]
Press release 10/19/2021
UNICEF medical supplies arrive in Kabul to treat rising cases of acute watery diarrhoea in Afghanistan
Press release 10/18/2021
House-to-house polio vaccination set to recommence across Afghanistan in November
[See Milestones above for detail]
Unitaid [to 23 Oct 2021]
https://unitaid.org/
Featured News
No new digest content identified.
Vaccine Equity Cooperative [nee Initiative] [to 23 Oct 2021]
https://vaccineequitycooperative.org/news/
News
No new digest content identified.
Vaccination Acceptance & Demand Initiative [Sabin) [to 23 Oct 2021]
https://www.vaccineacceptance.org/
Announcements
No new digest content identified.
Vaccine Confidence Project [to 23 Oct 2021]
http://www.vaccineconfidence.org/
News, Research and Reports
Coronavirus global impact
Launched April 2, 2020 and recurring every 3 days, Premise Data is utilizing its global network of Contributors to assess economic, social, and health sentiment surrounding the coronavirus (COVID-19).
Vaccine Education Center – Children’s Hospital of Philadelphia [to 23 Oct 2021]
http://www.chop.edu/centers-programs/vaccine-education-center
News
Vaccine Update Newsletter – September 2021
Vaccine Update is our monthly email newsletter that will keep you up to date on current vaccine-related issues.
Wellcome Trust [to 23 Oct 2021]
https://wellcome.ac.uk/news
News and reports
News
Wellcome appoints first climate director
18 October 2021
Alan Dangour, an expert in the impact of climate change on food systems, is to join Wellcome as our first director of climate and health.
The Wistar Institute [to 23 Oct 2021]
https://www.wistar.org/news/press-releases
Press Releases
No new digest content identified.
WFPHA: World Federation of Public Health Associations [to 23 Oct 2021]
https://www.wfpha.org/
Latest News
Pandemic Treaty: A Public Health View
News
Oct 20, 2021
The World Health Assembly (WHA) will meet in a special session from 29 November – 1 December 2021 to consider the benefits of developing a World Health Organisation (WHO) convention, agreement or another international instrument on pandemic preparedness and response.
As countries are preparing for discussions at the special session, the Global Health Centre (GHC) at the Graduate Institute of International and Development Studies in Geneva offers to support governments and other actors with knowledge and evidence in the process. This is part of a broader project exploring options and benefits of a pandemic treaty, which includes also, inter alia, a series of policy papers by lead experts, dialogue with diplomats, policymakers, and stakeholders.
Against this backdrop, the WFPHA and the GHC called for a meeting for WFPHA members and all allied partners on options and benefits of a pandemic treaty. The meeting took place in a virtual format on 20 October 2021.
The meeting was comprised of two sections: An introductory presentation on key global and public health/role of civil society aspects of the subject matter by the GHC and the WFPHA, followed by a Q&A session. The second section was an informal exchange and discussion between participants under the Chatham House rule. The knowledge gained, and the informal dialogues convened can contribute to WFPHA’s members and partners’ cooperation with governmental and non-state actors on the subject matter and may lead to a follow-up statement.
During the meeting, a Guide on key aspects and frequently asked questions on a pandemic treaty developed at the GHC was made available to the registered participants.
World Bank [to 23 Oct 2021]
http://www.worldbank.org/en/news/all
Selected News, Announcements
Food Security and COVID-19
Oct. 21, 2021 – An increasing number of countries are facing growing levels of acute food insecurity, reversing years of development gains. Even before COVID-19 reduced incomes and disrupted supply chains…
Date: October 21, 2021 Type: Brief
Rolling out COVID-19 Vaccines in Malawi Amid Hesitancy and Supply Challenges
LILONGWE, October 19, 2021— One early morning in August, Priscilla Pahuwa walked into St. Montfort hospital in Nchalo, Chikwawa District, to get the COVID-19 vaccine. She was eager to receive the vaccine…
Date: October 19, 2021 Type: Feature Story
Arrival of 4.73 Million Doses of WB-funded Vaccines Boosts Drive Against COVID-19 in the Philippines
MANILA, October 19, 2021 — To date, 4.73 million doses of vaccines out of 13 million doses procured by the country under the Philippines US$500 million COVID-19 Emergency Response Project Additional Financing…
Date: October 19, 2021 Type: Press Release
World Customs Organization – WCO [to 23 Oct 2021]
http://www.wcoomd.org/
Latest News – Selected Items
No new digest content identified.
World Organisation for Animal Health (OIE) [to 23 Oct 2021]
https://www.oie.int/en/media/news/
Press Releases, Statements
Building resilience against agro-terrorism and agro-crime
13 October 2021
The World Organisation for Animal Health (OIE), the Food and Agriculture Organization of the United Nations (FAO), and the International Criminal Police Organization (INTERPOL) are partners in an international project to build sustainable global resilience against animal health emergencies caused by agro-terrorism and agro-crime.
Established in October 2018, the project aims to foster coordination at the national, regional, and international levels. It focuses on regions where the previous work of the three organisations has identified gaps in various aspects of emergency management that may make countries vulnerable to agro-crime and agro-terrorism. The target regions include the Middle East, North Africa and South-East Asia. However, while the project concentrates on these regions, its outputs will be relevant to all countries worldwide.
To ensure that the resulting capacity building is fit for purpose, the project is currently assessing the global situation for emergency management by identifying areas that are vulnerable to agro-crime and agro-terrorism; understanding the cost-effectiveness of investing in preparedness; and using OIE, FAO and INTERPOL tools to examine emergency management, including the relationship between the law enforcement and veterinary sectors…
WTO – World Trade Organisation [to 23 Oct 2021]
http://www.wto.org/english/news_e/news_e.htm
WTO News and Events
Coordinated global response key to MSMEs’ post-pandemic economic recovery — DDG Zhang
22 October 2021
Deputy Director-General Xiangchen Zhang highlighted the importance of targeted policies and a coordinated global response to mitigate the impact of the COVID-19 pandemic on micro, small and medium-sized enterprises (MSMEs). DDG Zhang was speaking at a workshop organized by the WTO Chairs Programme at the University of Mauritius on 22 October. Noting that “MSMEs are the backbone of many economies”, he called on WTO members to “foster a transparent, inclusive, non-discriminatory, and predictable global trade environment that supports and enhances MSMEs’ involvement in international trade.” His remarks are below.
WHO, WIPO, WTO update information note on integrated health, trade, IP response to COVID-19
22 October 2021
On 22 October 2021, the World Health Organization (WHO), the World Intellectual Property Organization (WIPO) and the WTO launched an update of the extract “Integrated health, trade and IP approach to respond to the COVID-19 pandemic”. This extract, from the second edition of the Trilateral Study Promoting Access to Medical Technologies and Innovation, maps the challenges posed by the COVID-19 pandemic in relation to the integrated health, trade and intellectual property policy framework set out in the Trilateral Study.
::::::
ARM [Alliance for Regenerative Medicine] [to 23 Oct 2021]
https://alliancerm.org/press-releases/
Selected Press Releases
No new digest content identified.
BIO [to 23 Oct 2021]
https://www.bio.org/press-releases
Press Releases, Letters, Testimony, Comments [Selected]
No new digest content identified.
DCVMN – Developing Country Vaccine Manufacturers Network [to 23 Oct 2021]
http://www.dcvmn.org/
News; Upcoming events
No new digest content identified.
ICBA – International Council of Biotechnology Associations [to 23 Oct 2021]
https://internationalbiotech.org/news/
News
No new digest content identified.
IFPMA [to 23 Oct 2021]
http://www.ifpma.org/resources/news-releases/
Selected Press Releases, Statements, Publications
As COVID-19 vaccine output estimated to reach over 12 billion by year end and 24
19 October 2021
This month, COVID-19 vaccine manufacturing output will pass the 9.3 billion dose mark. The biopharmaceutical industry renews its May commitment to the G20 to collaborate with governments to support effective solutions to urgently address vaccine equity.
At current production rates, swiftly rolling out vaccines to those who still need them looks achievable, if the political will, planning and collective action efforts are redoubled. The G20 can play a critical role in focusing political attention on ramping up dose sharing, addressing bottlenecks in the supply chain and supporting country preparedness.
With COVID-19 vaccine supplies on track to outstrip global demand and voluntary collaborations with innovative vaccine manufacturers set to change Africa’s vaccine manufacturing landscape in years to come, initiatives such as the proposed Intellectual Property TRIPS waiver are a distraction. All efforts should be directed towards advancing vaccinations.
International Generic and Biosimilar Medicines Association [IGBA]
https://www.igbamedicines.org/
News
IGBA Biosimilars Committee White Paper: A Biosimilar medicines Access Policy Blueprint (October 2021)
Effective Strategies to Advance Access to Biologic Therapies for Non-Communicable Diseases
October 2021 :: 32 pages PDF: https://www.globalbiosimilarsweek.org/2021/doc/A-Biosimilar-medicines-Access-Policy-Blueprint-IGBA.pdf
International Alliance of Patients’ Organizations – IAPO [to 23 Oct 2021]
https://www.iapo.org.uk/news/topic/6
Press and media [Selected]
Registration is now open for the 3rd Asia-Pacific Patients Congress (APPC 2021)
Monday, 18 October 2021
We are delighted to invite you to register for our upcoming virtual 3rd Asia-Pacific Patients Congress (APPC 2021) taking place on 16 -17 November 2021. This flagship event will once again bring together our regional membership of expert patients with a variety of high-level healthcare stakeholders…
Sample Program Element [Day 2]
Meaningful patient engagement in co-creation in medicines and health devices R&D – moving from tokenism to meaningful engagement at all levels
The COVID-19 ‘fast-track’ vaccines development process has educated many patients on how clinical trials are conducted. Asia-Pacific patients are now more aware of the opportunities and challenges faced by R&D teams. This session will invite experts to showcase how meaningful patient engagement in clinical R&D settings is undertaken now. We will also invite PPI Japan EUPATI’s national platform in Japan to show how meaningful patient engagement in clinical trials is conducted in Japan.
PhRMA [to 23 Oct 2021]
http://www.phrma.org/
Latest News [Selected]
In Honor of Visionary Leader, PhRMA Announces the Kenneth C. Frazier PhRMA Scholars Program
WASHINGTON, D.C. (October 21, 2021) – In honor of retiring PhRMA board member and visionary industry leader Ken Frazier, PhRMA today announced The Kenneth C. Frazier PhRMA Scholars Program to provide college scholarships to academically high achieving, low-income students in Washington, D.C. and in Ken’s hometown of Philadelphia, Pennsylvania. Frazier, currently executive chairman of Merck & Co, Inc., has served on the PhRMA board since 2011…