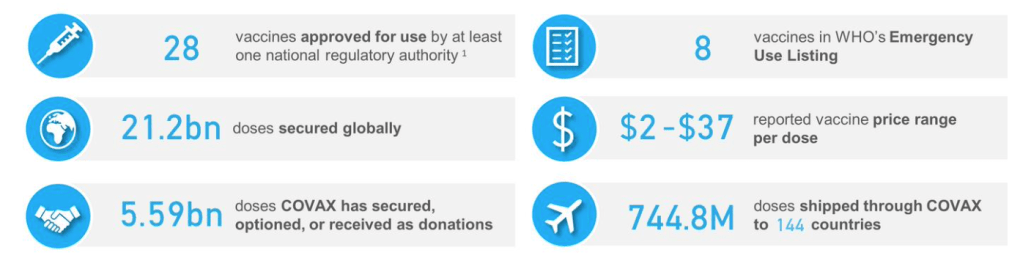
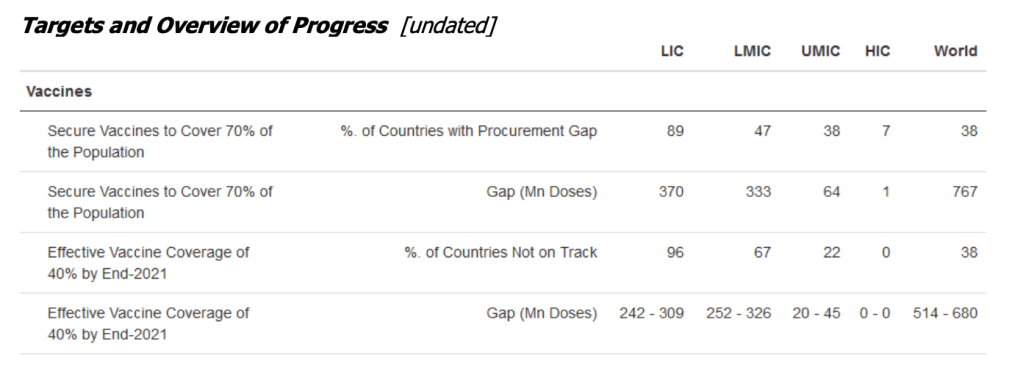
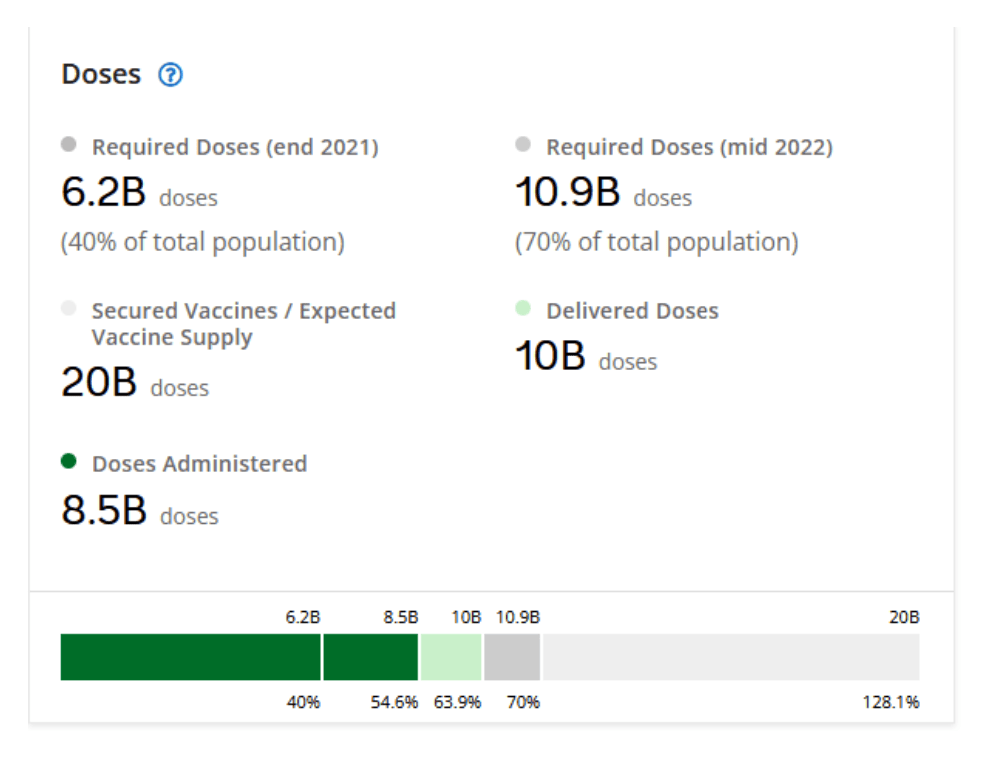
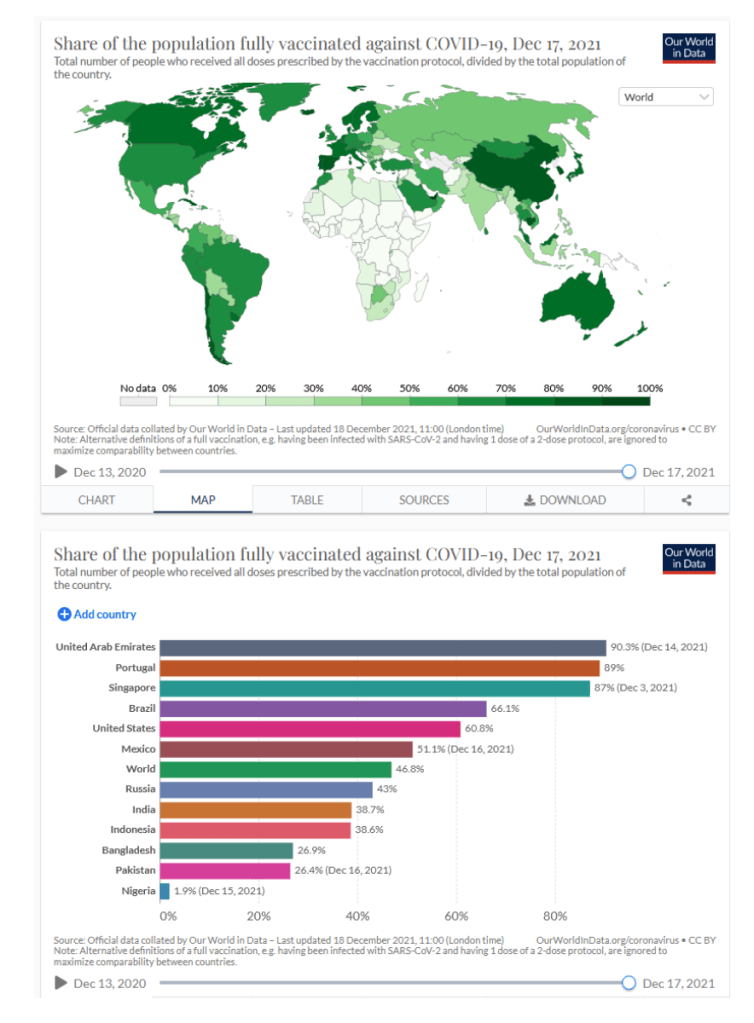
::::::
Organization Announcements
Editor’s Note:
Careful readers will note that the number and range of organizations now monitored in our Announcements section below has grown as the impacts of the pandemic have spread across global economies, supply chains and programmatic activity of multilateral agencies and INGOs.
Paul G. Allen Frontiers Group [to 18 Dec 2021]
https://alleninstitute.org/news-press/
News
Hundreds of small molecules known as neuromodulators might influence how we learn
December 17, 2021
New models that capture how the brain retains information could also improve machine learning
Press Release
Allen Institute announces Rui Costa as next President and Chief Executive Officer
December 16, 2021
The Allen Institute today named Rui Costa, D.V.M., Ph.D., as its next president and chief executive officer. Costa comes to the Allen Institute from the Mortimer B. Zuckerman Mind Brain Behavior Institute at Columbia University in New York where he has served as CEO since 2017, and currently serves on the Allen Institute’s Brain Science Scientific Advisory Council…
BARDA – U.S. Department of HHS [to 18 Dec 2021]
https://www.phe.gov/about/barda/Pages/default.aspx
News
No new digest content identified.
BMGF – Gates Foundation [to 18 Dec 2021]
https://www.gatesfoundation.org/ideas/media-center
Press Releases and Statements
No new digest content identified.
Bill & Melinda Gates Medical Research Institute [to 18 Dec 2021]
https://www.gatesmri.org/
The Bill & Melinda Gates Medical Research Institute is a non-profit biotech organization. Our mission is to develop products to fight malaria, tuberculosis, and diarrheal diseases—three major causes of mortality, poverty, and inequality in developing countries. The world has unprecedented scientific tools at its disposal; now is the time to use them to save the lives of the world’s poorest people
No new digest content identified.
CARB-X [to 18 Dec 2021]
https://carb-x.org/
News
No new digest content identified.
Center for Vaccine Ethics and Policy – GE2P2 Global Foundation [to 18 Dec 2021]
https://centerforvaccineethicsandpolicy.net/
News/Analysis/Statements
:: Past weekly editions and posting of all segments of Vaccines and Global Health: The Week in Review are available here.
:: Informed Consent: A Monthly Review – December 2021 is now posted here
CEPI – Coalition for Epidemic Preparedness Innovations [to 18 Dec 2021]
http://cepi.net/
Latest News
CEPI statement: CEO welcomes Emergency Use Listing for NVX-CoV2373
CEPI-supported vaccine is the first protein-based vaccine to receive EUL, a prerequisite for distribution through COVAX.
17 Dec 2021
CEPI partners with Affinivax to develop a novel COVID-19 vaccine to target variants
New partnership will advance the development of a novel vaccine that could provide broad protection against SARS-CoV-2 variants, and potentially other betacoronaviruses… EPI will provide funding of up to $4.5 million to support the initial development of a vaccine candidate based on Affinivax’s innovative Multiple Antigen Presenting System (MAPSTM) technology platform.
13 Dec 2021
DARPA – Defense Advanced Research Projects Agency [to 18 Dec 2021
https://www.darpa.mil/news
News
No new digest content identified.
Duke Global Health Innovation Center [to 18 Dec 2021]
https://dukeghic.org/
Our Blog
No new digest content identified.
EDCTP [to 18 Dec 2021]
http://www.edctp.org/
The European & Developing Countries Clinical Trials Partnership (EDCTP) aims to accelerate the development of new or improved drugs, vaccines, microbicides and diagnostics against HIV/AIDS, tuberculosis and malaria as well as other poverty-related and neglected infectious diseases in sub-Saharan Africa, with a focus on phase II and III clinical trials
News
No new digest content identified.
Emory Vaccine Center [to 18 Dec 2021]
http://www.vaccines.emory.edu/
Vaccine Center News
No new digest content identified.
European Vaccine Initiative [to 18 Dec 2021]
http://www.euvaccine.eu/
Latest News, Events
No new digest content identified.
Fondation Merieux [to 18 Dec 2021]
http://www.fondation-merieux.org/
News, Events
No new digest content identified.
Gavi [to 18 Dec 2021]
https://www.gavi.org/
News Releases
No new digest content identified.
GHIT Fund [to 18 Dec 2021]
https://www.ghitfund.org/newsroom/press
GHIT was set up in 2012 with the aim of developing new tools to tackle infectious diseases that
No new digest content identified.
Global Fund [to 18 Dec 2021]
https://www.theglobalfund.org/en/news/
News & Stories
News
Ethics Resources for Country Coordinating Mechanisms: New e-learning Module
17 December 2021
A new e-learning module has been launched to help Country Coordinating Mechanisms (CCM) maintain high standards of ethics and integrity in their work and decision making. The new module focuses on Duty of Care (including Oversight) and Accountability (including Transparency) and is available on iLearn in English. It will soon be available in French and Spanish. This fifth module concludes the CCM Code of Conduct training series.
The e-learning module can be found on the Country Coordinating Mechanism Ethics section, along with the other code of conduct modules for CCM members and related information.
Global Research Collaboration for Infectious Disease Preparedness [GloPID-R] [to 18 Dec 2021]
https://www.glopid-r.org/news/
News
No new digest content identified.
Hilleman Laboratories [to 18 Dec 2021]
http://www.hillemanlabs.org/
News & Insights
No new digest content identified.
HHMI – Howard Hughes Medical Institute [to 18 Dec 2021]
https://www.hhmi.org/news
Press Room
Research Dec 15 2021
Flies Navigate Using Complex Mental Math
For the first time, scientists have shown exactly how a fly brain creates a mental map of the body’s movement through the world.
Human Vaccines Project [to 18 Dec 2021]
http://www.humanvaccinesproject.org/
News
No new digest content identified.
IAVI [to 18 Dec 2021]
https://www.iavi.org/newsroom
Latest News
No new digest content identified.
International Coalition of Medicines Regulatory Authorities [ICMRA]
http://www.icmra.info/drupal/en/news
Selected Statements, Press Releases, Research
No new digest content identified.
ICRC [to 18 Dec 2021]
https://www.icrc.org/en/whats-new
Selected News Releases, Statements, Reports
ICRC collaborates with artists in urgent call for equitable access to COVID-19 vaccine
The ICRC has collaborated with artists from Asia-Pacific on illustrations to emphasize the importance and call for the equitable access to COVID-19 vaccines.
16-12-2021 | Article
IFFIm
http://www.iffim.org/
Press Releases/Announcements
Lessons on COVID-19 from Africa: Q&A with Hassatou N’Sele
17 Dec 2021
Hassatou N’Sele joined IFFIm’s Board of Directors on 1 July 2021. As the Treasurer of the African Development Bank Group (AfDB) based in Abidjan, Cote d’Ivoire, she leads the expansion of the AfDB capital markets activities across the globe. Ms N’Sele is a Senegalese citizen. Tell us about your life. What experiences and people shaped you as a per…
The Wild Side of Innovative Finance: Q&A with Monique Barbut
17 Dec 2021
Monique Barbut joined IFFIm’s Board of Directors in July 2021. Currently the President of the World Wildlife Fund France, Ms Barbut has spent her extensive career in public service, playing a key role in environmental and financial global negotiations at the U.N.
Channeling private sector participation to optimise results: Q&A with Ingrid van Wees
17 Dec 2021
Ingrid van Wees joined the IFFIm board on 1 October 2021. She is currently the Vice President for Finance and Risk Management of the Asian Development Bank (ADB), based in Manila, the Philippines.
IFRC [to 18 Dec 2021]
http://media.ifrc.org/ifrc/news/press-releases/
Selected Press Releases, Announcements
16/12/2021
DRC: Media statement on the end of the 13th Ebola outbreak
Beni/Kinshasa/Nairobi/Geneva, 16 December 2021—The Red Cross of the Democratic Republic of Congo (DRC) joins the International Federation of Red Cross and Red Crescent Societies (IFRC) in celebrating the end of the 13th Ebola outbreak in the country.
The Minister of Public Health, Hygiene and Prevention of the DRC, officially declared, this 16 December 2021, the end of the 13th outbreak of Ebola Virus Disease which resurfaced on 8 October 2021, in the health zone of Beni in the province of North Kivu.
This declaration comes 42 days after the last patient tested negative.
A cumulative 11 cases were recorded during this 13th outbreak, including 8 deaths and 2 cured…
16/12/2021
Red Cross Red Crescent reaching 1.5 million people on the move in MENA, yet millions are left without support
Institut Pasteur [to 18 Dec 2021]
https://www.pasteur.fr/en/press-area
Press Documents
Press release 06.12.2021
Chronic obstructive pulmonary disease: a genetic mutation confirmed as predisposing factor
In 2019, the WHO positioned chronic obstructive pulmonary disease (COPD) third in the global ranking of causes of death…
IOM / International Organization for Migration [to 18 Dec 2021]
http://www.iom.int/press-room/press-releases
News – Selected
News
15 Dec 2021
IOM Launches COVID-19 Vaccination Campaign for Migrants in Yemen
Aden – The International Organization for Migration (IOM) has begun a COVID-19 vaccination campaign for migrants stranded in Yemen, aiming to inoculate around 7,500 people at its Migrant Response Points in Aden and Ma’rib. IOM continues to advocate for more efforts to protect vulnerable populations by ramping up vaccination efforts for people on the move.
“We welcome the Government’s commitment to protecting migrants against COVID-19 and immunizing people on the move is key to combatting the spread of the disease,” said IOM’s Chief of Mission, Christa Rottensteiner. “There are still not enough doses to protect everyone in Yemen from this disease. More support from the international community to supply the country with enough vaccines will save lives.”…
So far this year, more than 135,000 people have been reached with COVID-19 awareness sessions and over 400,000 people have been screened for COVID-19 at IOM-supported health facilities throughout the country.
IOM’s vaccination campaign for migrants in Yemen is implemented in partnership with the Ministry of Health and the World Health Organization with support by contributions from the governments of Germany, Finland and EU Humanitarian Aid.
ISC / International Science Council [to 18 Dec 2021]
https://council.science/current/
ISC is a non-governmental organization with a unique global membership that brings together 40 international scientific Unions and Associations and over 140 national and regional scientific organizations including Academies and Research Councils.
News
No new digest content identified.
IVAC [to 18 Dec 2021]
https://www.jhsph.edu/research/centers-and-institutes/ivac/index.html
Updates; Events
No new digest content identified.
IVI [to 18 Dec 2021]
http://www.ivi.int/
IVI News & Announcements
IVI to establish a European regional office in Sweden
IVI and the Government Offices of Sweden signed a Memorandum of Understanding today to establish the office in Stockholm, creating a European hub for global health research and innovation
December 17, 2021
Johns Hopkins Center for Health Security [to 18 Dec 2021]
https://www.centerforhealthsecurity.org/news/center-news/
Center News
New Report: Integrating Primary Care and Public Health to Save Lives and Improve Practice During Public Health Crises: Lessons from COVID-19
December 14, 2021
Today, the Johns Hopkins Center for Health Security at the Bloomberg School of Public Health released a new report finding that the failure to bring primary care providers into a frontline role as responders to the COVID-19 pandemic, alongside the public health system, resulted in many missed opportunities to provide better quality care, faster testing, more effective contact tracing, greater acceptance of vaccination, and better communication with patients.
The new report, Integrating Primary Care and Public Health to Save Lives and Improve Practice During Public Health Crises: Lessons from COVID-19, also identified other unrealized benefits that could have helped, had there been better integration of primary care, public health, and community-based organizations: 1) Greater support for the public health response, thereby easing the burden on overstretched public health personnel; and 2) ability to access primary care’s reach to amplify public health messaging…
MSF/Médecins Sans Frontières [to 18 Dec 2021]
http://www.msf.org/
Latest [Selected Announcements]
Brazil
Venezuelan migrants left without healthcare, shelter and services
Project Update 14 Dec 2021
National Academy of Medicine – USA [to 18 Dec 2021]
https://nam.edu/programs/
Selected News/Programs/Events
No new digest content identified.
National Academy of Sciences – USA [to 18 Dec 2021]
http://www.nasonline.org/news-and-multimedia/
News
No new digest content identified.
National Vaccine Program Office – U.S. HHS [to 18 Dec 2021]
https://www.hhs.gov/vaccines/about/index.html
Upcoming Meetings/Latest Updates
No new digest content identified.
NIH [to 18 Dec 2021]
http://www.nih.gov/news-events/news-releases
News Releases
NIH scientists urge pursuit of universal coronavirus vaccine
December 16, 2021 — A growing body of scientific evidence suggests that novel coronaviruses will continue to have potential to emerge as a threat to humans.
DM Morens, et al. Universal coronavirus vaccines—an urgent need. The New England Journal of Medicine. DOI: 10.1056/NEJMp2118468(link is external) (2021).
[See Milestones/Perspectives above for detail]
OECD [to 18 Dec 2021]
http://www.oecd.org/newsroom/publicationsdocuments/bydate/
Newsroom
No new digest content identified.
PATH [to 18 Dec 2021]
https://www.path.org/media-center/
Press Releases
No new digest content identified.
Sabin Vaccine Institute [to 18 Dec 2021]
http://www.sabin.org/updates/pressreleases
Statements and Press Releases
No new digest content identified.
UNAIDS [to 18 Dec 2021]
http://www.unaids.org/en
Selected Press Releases/Reports/Statements
13 December 2021
Key population participation in HIV decision-making varies
UNHCR Office of the United Nations High Commissioner for Refugees [to 18 Dec 2021]
http://www.unhcr.org/en-us/media-centre.htmlS
Selected News Releases, Announcements
No new digest content identified.
UNICEF [to 18 Dec 2021]
https://www.unicef.org/media/press-releases
Press Releases, News Notes, Statements [Selected]
Statement
12/17/2021
Even as Omicron variant takes hold, school closures must be a measure of last resort
Statement by UNICEF Executive Director Henrietta Fore
Unitaid [to 18 Dec 2021]
https://unitaid.org/
Featured News
17 December 2021
Unitaid launches call for proposals to prevent hepatitis C in the most marginalised and at-risk groups
Geneva – An estimated 58 million people worldwide are infected with hepatitis C, a blood-borne virus that can lead to serious liver disease. This virus, which causes an estimated 300,000 deaths each year, affects people who inject drugs and incarcerated populations at much higher rates.
Unitaid is therefore seeking to fund innovative projects that will expand access to new tools or under-utilised interventions to prevent and treat hepatitis C, with a particular focus on these key groups in low- and middle-income countries…
16 December 2021
Former French Minister Marisol Touraine reelected chair of the Unitaid Executive Board; Unitaid on track to deliver its new strategy for 2022-26
USAID [to 18 Dec 2021]
https://www.usaid.gov/news-information/press-releases/2021
Selected Press Releases, Statements, Announcements
Administrator Samantha Power Holds Meetings on COVID-19 Vaccine Access and Delivery Efforts
December 17, 2021
This week, USAID Administrator Samantha Power held a series of high-level meetings with key United States and world health experts to discuss the recently announced whole-of-government effort, the Initiative for Global Vaccine Access (Global VAX). Vaccinating the world is the best way to save lives, prevent future variants that threaten the health of communities around the world, including Americans, and undermine the global economic recovery. As more vaccine supply flows to low and middle income countries, the United States is redoubling efforts to help countries efficiently and effectively receive, distribute, and administer doses. Global VAX focuses on accelerating global efforts to get shots in arms —particularly in sub-Saharan Africa—and to enhance international coordination to rapidly overcome barriers to vaccine delivery.
Vaccine Equity Cooperative [nee Initiative] [to 18 Dec 2021]
https://vaccineequitycooperative.org/news/
News
No new digest content identified.
Vaccination Acceptance & Demand Initiative [Sabin) [to 18 Dec 2021]
https://www.vaccineacceptance.org/
Announcements
Focusing on Marginalized Communities
Get to Know Two of Our 2021 Social and Behavioral Research Grant Partners
December 14, 2021
By: Abigail Quinn, BA, Deeva Agravat, MSc, Kate Hopkins, PhD, MPH
Vaccine Confidence Project [to 18 Dec 2021]
http://www.vaccineconfidence.org/
News, Research and Reports
No new digest content identified.
Vaccine Education Center – Children’s Hospital of Philadelphia [to 18 Dec 2021]
http://www.chop.edu/centers-programs/vaccine-education-center
News
No new digest content identified.
Wellcome Trust [to 18 Dec 2021]
https://wellcome.ac.uk/news
News. Opinion, Reports
News
How to prevent another major pandemic
15 December 2021
The world could have been much better prepared for Covid-19. So now we must ask: How well prepared are we going to be for the next pandemic?
The Wistar Institute [to 18 Dec 2021]
https://www.wistar.org/news/press-releases
Press Releases
No new digest content identified.
WFPHA: World Federation of Public Health Associations [to 18 Dec 2021]
https://www.wfpha.org/
Latest News – Blog
Facing Leadership that Kills!
Dec 15, 2021 | News
Facing Leadership that Kills! An Article by Public Health Leadership Coalition’s Member – Dr. Alejandro R. Jadad Despite the growing number of reports describing myriad ways in which leaders failed the populations they were meant to protect during the COVID-19…
World Bank [to 18 Dec 2021]
http://www.worldbank.org/en/news/all
Selected News, Announcements
Ukraine to Expand COVID-19 Vaccination, with Additional World Bank Financing
WASHINGTON, December 10, 2021 – The World Bank’s Board of Executive Directors approved today $150 million in Additional Financing for the Ukraine Emergency COVID-19 Response and Vaccination Project…
Date: December 10, 2021 Type: Press Release
Learning Losses from COVID-19 Could Cost this Generation of Students Close to $17 Trillion in Lifetime Earnings
World Bank-UNESCO-UNICEF report lays out the magnitude of the education crisis WASHINGTON, DC, Dec. 6, 2021—This generation of students now risks losing $17 trillion in lifetime earnings in present…
Date: December 06, 2021 Type: Press Release
The State of the Global Education Crisis: A Path to Recovery
The global disruption to education caused by the COVD-19 pandemic is without parallel and the effects on learning are severe. The crisis brought education systems across the world to a halt, with school…
Date: December 03, 2021 Type: Publication
World Organisation for Animal Health (OIE) [to 18 Dec 2021]
https://www.oie.int/en/media/news/
Press Releases, Statements
No new digest content identified.
WTO – World Trade Organisation [to 18 Dec 2021]
http://www.wto.org/english/news_e/news_e.htm
WTO News and Events
Members continue discussions on IP COVID-19 response as high-level engagement intensifies
16 December 2021
WTO members agreed to continue their discussions on a common intellectual property (IP) response to COVID-19 amid the ongoing high-level political dialogue aimed at finding a consensus-based outcome. At a formal meeting of the Council for Trade-Related Aspects of Intellectual Property Rights (TRIPS) on 16 December, members reported on the bilateral meetings and small group consultations held following the postponement of the 12th WTO Ministerial Conference and reiterated their willingness and intention to intensify their engagement with each other.
[See Milestones/Perspectives above for detail]
WTO updates note on trade in medical goods in the context of COVID-19
16 December 2021
The WTO Secretariat has published an update of the information note on trade in medical goods in the context of tackling COVID-19, which was first issued in April 2020, a few months after the World Health Organization declared COVID-19 a public health emergency of international concern. The update looks at developments in the first half of 2021.
The updated note indicates that in the first half of 2021, trade in medical goods maintained solid growth, comprising 6.1 per cent of total world trade compared to 5.4 per cent for the second half of 2019, just before the pandemic outbreak.
Trade in medical goods grew by 12.4 per cent in the first half of 2021 compared with the same period in 2020. This increase was slower than the year-on-year growth for the second half of 2020, at 17.1 per cent, but it is an increase of 31 per cent compared to the first half of 2019. It is also a continuation of the strong growth (16 per cent) recorded in 2020.
The update features a case study on the critical products needed for administering COVID-19 vaccines. This highlights that as vaccination numbers increased, the highest year-on-year growth (34.8 per cent) was for medical supplies, including items critical for administering vaccines (rubber gloves, syringes and needles).
Trade in testing materials and diagnostic reagents also remained high, growing by 54.5 per cent in the first half of 2021 compared to the same period of 2020.
The first information note on “Trade in medical goods in the context of tackling COVID-19” was issued on 3 April 2020, with previous updates issued on 20 December 2020 and 30 June 2021. The updated note can be found here.
::::::
ARM [Alliance for Regenerative Medicine] [to 18 Dec 2021]
https://alliancerm.org/press-releases/
Selected Press Releases
No new digest content identified.
BIO [to 18 Dec 2021]
https://www.bio.org/press-releases
Press Releases, Letters, Testimony, Comments [Selected]
No new digest content identified.
DCVMN – Developing Country Vaccine Manufacturers Network [to 18 Dec 2021]
http://www.dcvmn.org/
News; Upcoming events
16 December 2021
Press release: 11 billion COVID-19 vaccines produced in 2021 has resulted in the biggest immunization campaign in human history and 2022 will require more and better vaccine redistribution and innovation
[See Milestones/Perspectives above for detail]
ICBA – International Council of Biotechnology Associations [to 18 Dec 2021]
https://internationalbiotech.org/news/
News
No new digest content identified.
IFPMA [to 18 Dec 2021]
http://www.ifpma.org/resources/news-releases/
Selected Press Releases, Statements, Publications
11 billion COVID-19 vaccines produced in 2021 has resulted in the biggest immunization campaign in human history and 2022 will require more and better vaccine redistribution and innovation
Published on: 16 December 2021
[See Milestones/Perspectives above for detail]
Position paper – Synthetic follow-on products that reference biologically produced medicines
15 December 2021
What is the challenge?
In some countries, there is lack of clarity about how these products should be regulated….“Synthetic follow-on products that reference a biologically produced medicine are complex and should not be viewed as simple generics. Instead, they should be evaluated following an approach more aligned with that adopted for biosimilars.”
Technology Transfer: A Collaborative Approach to Improve Global Health
13 December 2021
International Generic and Biosimilar Medicines Association [IGBA]
https://www.igbamedicines.org/
News
No new digest content identified.
International Alliance of Patients’ Organizations – IAPO [to 18 Dec 2021]
https://www.iapo.org.uk/news/topic/6
Press and media [Selected]
Joint Statement On Patient Solidarity Day 2021
London, December 3rd, 2021 – On Patient Solidarity Day, the International Alliance of Patients’ Organizations (IAPO), the International Pharmaceutical Federation (FIP), the International Federation of Pharmaceutical Manufacturers and Associations (IFPMA), International Hospital Federation (IHF) and the International Council of Nurses (ICN) , join patients and families all over the world, in calling for governments and all health stakeholders to collaborate in the implementation of the WHO Flagship Global Patient Safety Action Plan 2021-2030 (GPSAP 2021-30) that was adopted at the 74th World Health Assembly this year.
PhRMA [to 18 Dec 2021]
http://www.phrma.org/
Latest News [Selected]
No new digest content identified.