Europe: COVID-19 Vaccines – Announcements/Regulatory Actions/Deployment
European Medicines Agency
News & Press Releases
News: EMA issues advice on use of Lagevrio (molnupiravir) for the treatment of COVID-19 (new)
CHMP, Last updated: 19/11/2021
News: EMA starts review of Paxlovid for treating patients with COVID-19 (new)
CHMP, Last updated: 19/11/2021
News: European Antibiotic Awareness Day: Fighting the silent pandemic (new)
Last updated: 18/11/2021
News: EMA receives application for marketing authorisation for Xevudy (sotrovimab) for treating patients with COVID-19 (new)
CHMP, PDCO, PRAC, Last updated: 18/11/2021
News: EMA receives application for conditional marketing authorisation of Novavax’s COVID-19 vaccine, Nuvaxovid (new)
CHMP, Last updated: 17/11/2021
::::::
European Centre for Disease Prevention and Control
https://www.ecdc.europa.eu/en
Latest Updates
Publication
Assessment of electronic health records for infectious disease surveillance
Technical report – 19 Nov 2021
News
Reported decrease in antibiotic consumption across EU/EEA during COVID-19 pandemic
Press release – 18 Nov 2021
Publication
Antimicrobial consumption in the EU/EEA (ESAC-Net) – Annual Epidemiological Report for 2020
Surveillance report – 18 Nov 2021
::::::
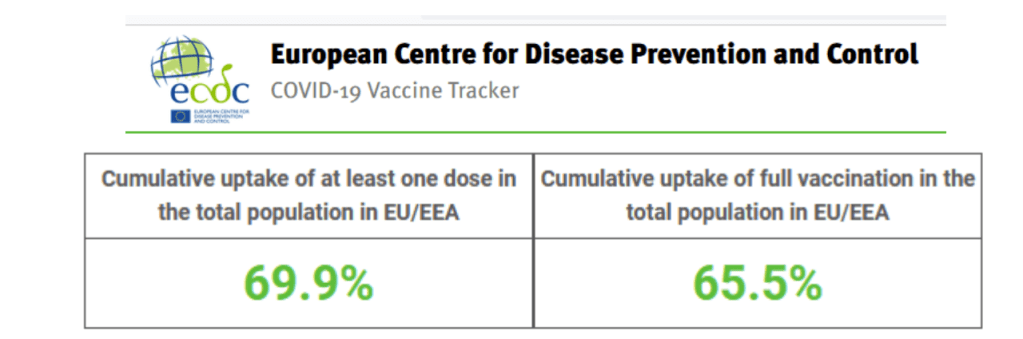
Accessed 20 Nov 2021
https://vaccinetracker.ecdc.europa.eu/public/extensions/COVID-19/vaccine-tracker.html#uptake-tab
::::::
European Commission
https://ec.europa.eu/commission/presscorner/home/en
No new digest content identified.