Featured Journal Content
Editor’s Note:
We anticipate that Gavi/COVAX will respond to elements of this report and we will present any response on our next edition.
The Lancet
Volume 397, ISSUE 10292,
P2322-2325, June 19, 2021
World Report
A beautiful idea: how COVAX has fallen short
Ann Danaiya Usher
Published:June 19, 2021 DOI:https://doi.org/10.1016/S0140-6736(21)01367-2
COVAX was meant to supply COVID-19 vaccines for all based on solidarity and equity. Instead, it relies on rich countries’ willingness to share their doses. Ann Danaiya Usher reports.
Launched 1 year ago, the COVAX facility was conceived as an “unparalleled and ambitious” attempt to create a global procurement mechanism to supply COVID-19 vaccines to all countries in the world. It was hailed as a “global, heroic effort” that would “transcend the limits of human ingenuity” to ensure that vaccine development progressed as fast as possible, at “a speed, scale, and access never before seen in human history”. Underlying everything, according to early descriptions by Gavi, the Vaccine Alliance, it was “single minded in its goal to ensure equitable access to COVID-19 vaccines”.
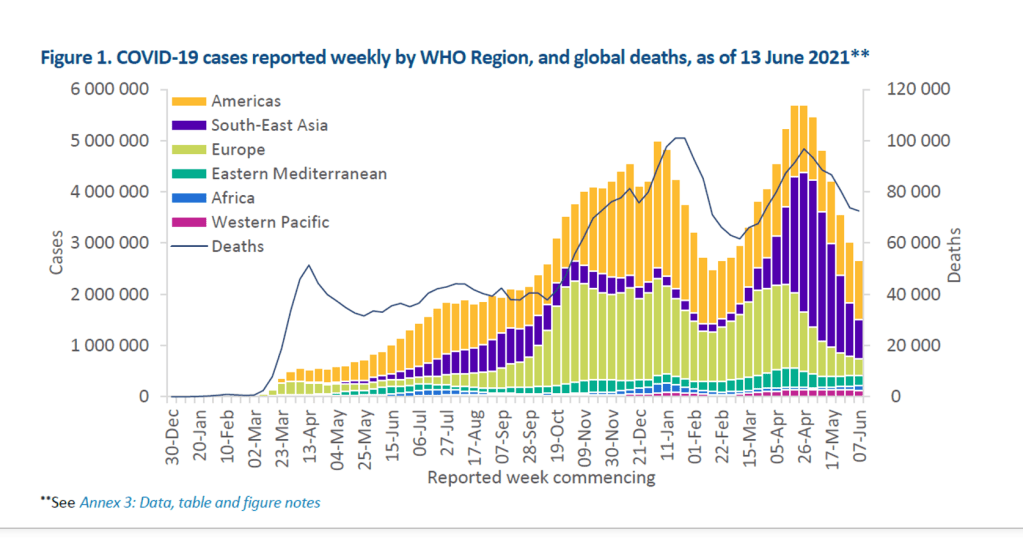
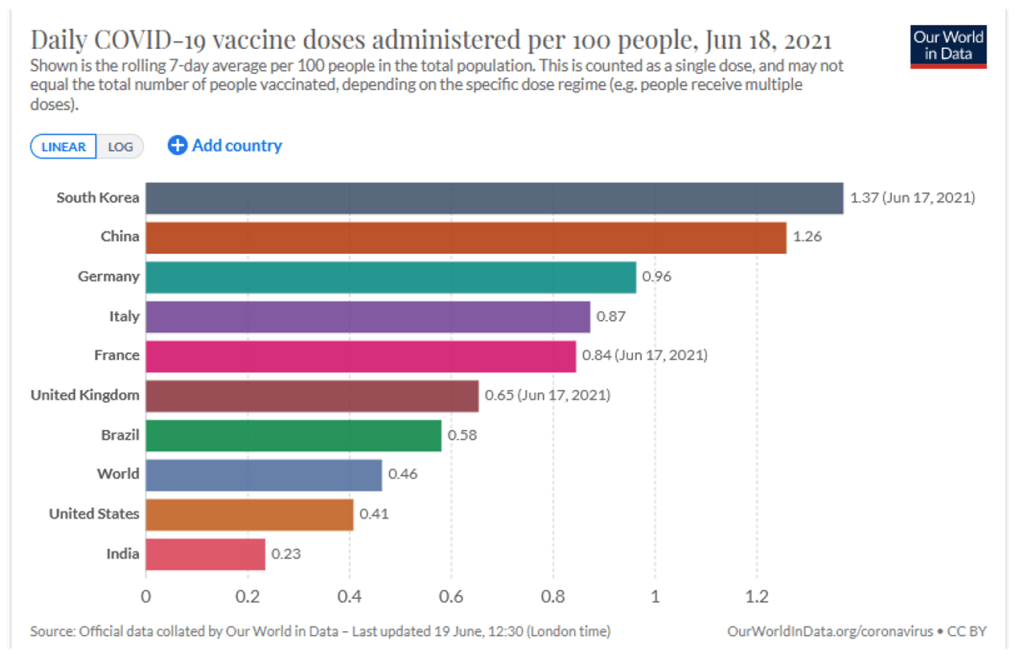
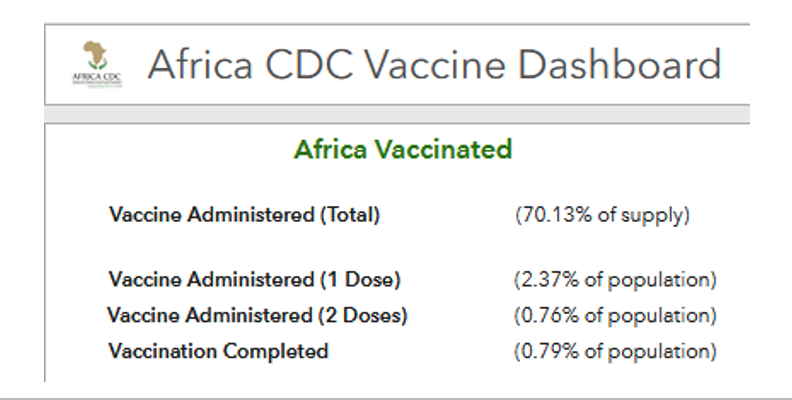
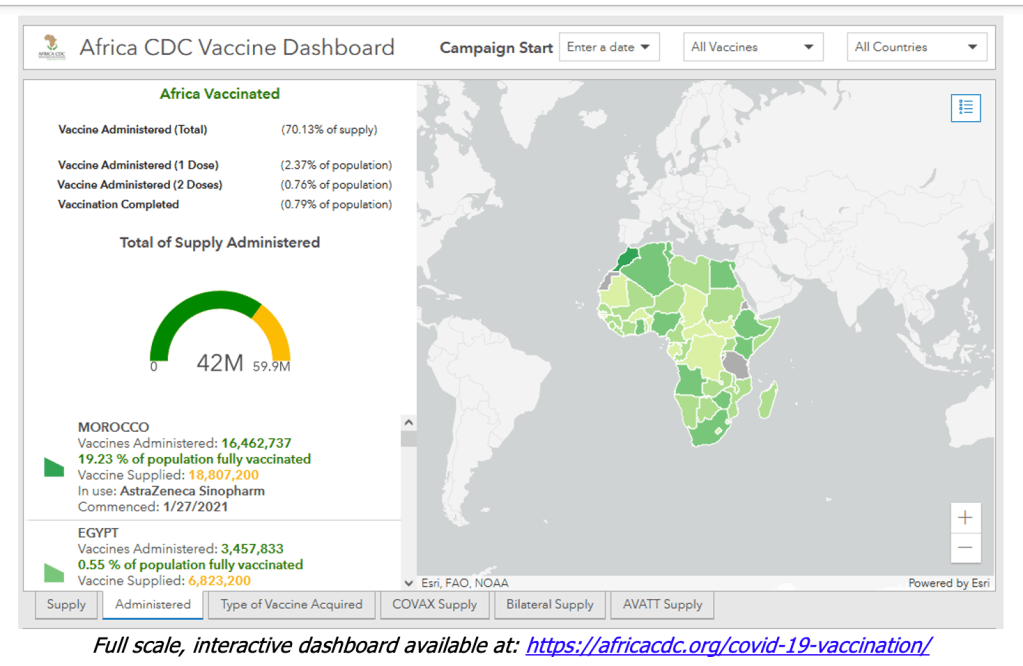
This vision has not come to pass. At the pledging summit for COVAX on June 2, 2021, hosted by Japan, Gavi finally reached its US$8·3 billion ask for the procurement and delivery of vaccines for the 92 eligible low-income and middle-income countries (LMICs) this year. However, even with full financing, the COVAX roll-out has moved much more slowly than that in high-income countries (HICs). Speaker after speaker at the summit lamented the gross inequity in access to vaccines. “Today, ten countries have administered 75% of all COVID-19 vaccines, but, in poor countries, health workers and people with underlying conditions cannot access them. This is not only manifestly unjust, it is also self-defeating”, UN secretary general António Guterres told the gathering. “COVAX has delivered over 72 million doses to 125 countries. But that is far less than 172 million it should have delivered by now.” Of the 2·1 billion COVID-19 vaccine doses administered worldwide so far, COVAX has been responsible for less than 4%.
”Born out of solidarity”
Gavin Yamey at Duke University (Durham, NC, USA) was part of a working group, convened by Gavi in early 2020, to discuss the design of COVAX. “It was a beautiful idea, born out of solidarity”, he said. “Unfortunately, it didn’t happen…Rich countries behaved worse than anyone’s worst nightmares.”
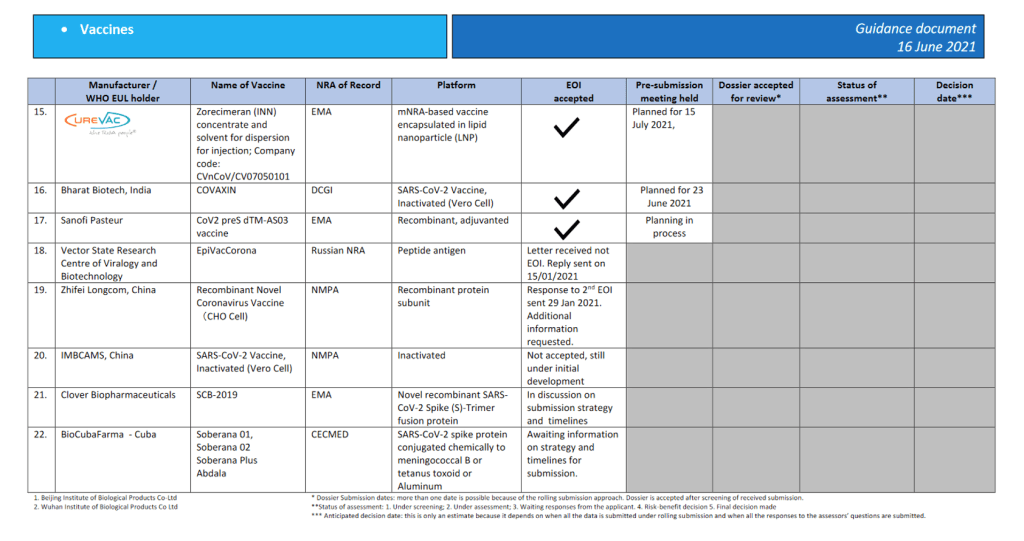
COVAX, managed by Gavi, along with the Coalition for Epidemic Preparedness Innovations and WHO, was designed to stand on two legs: one for HICs, which would pay for their own vaccines, and the other for 92 lower-income countries, whose doses would be financed by donor aid.
In the so-called self-financing leg of COVAX, HICs were asked to pay upfront by mid-September, 2020, for the option to buy vaccines for their own populations. The UK, for example, paid £71 million for 27 million doses from COVAX, and Canada paid CA$220 million for 15 million doses. Australia, New Zealand, Norway, and South Korea also bought vaccine options from COVAX as self-financing countries.
In the other leg of COVAX, vaccines for lower-income countries would be financed with donor grants through an Advance Market Commitment (AMC). The poorest of the 92 countries would receive them at no cost. Team Europe (led by Germany) and the USA have together provided US$5 billion to the COVAX AMC, Japan has given US$1 billion, and the UK, US$735 million. Most of these funds have been pledged only in the past few months.
The grand idea of COVAX was that the combination of these two funding streams—the self-financed part and the aid-financed AMC—would give the facility the means to invest in research and development of several promising vaccine candidates. Additionally, as a pooled procurement mechanism, COVAX would have the financial muscle as a buyer to drive down prices for all participants. Once any of the COVAX portfolio vaccines had successfully undergone clinical trials and proved themselves to be both safe and effective, both self-financing and AMC countries would be allocated vaccines at the same rate, proportional to their total population size.
COVAX would be “quite literally a lifeline” for self-financing countries that had not made any bilateral deals with vaccine manufacturers, Gavi’s chief executive officer Seth Berkley explained last autumn. However, by August, 2020, the USA had already entered into seven bilateral deals with six companies for more than 800 million doses, enough to vaccinate 140% of its population, according to the Duke University Launch and Scale Speedometer. The EU was close behind with access to half a billion doses secured through two deals. The UK had bought into five bilateral deals giving it access to 270 million doses, equivalent to 225% of its population. These early investments by rich countries in multiple vaccines secured them a place at the front of the queue. Because COVAX did not have the means to compete, it was pushed to the back.
Writing in a blog post earlier this year, Andrea Taylor, who manages the Launch and Scale Speedometer vaccine tracker, has said that “COVAX was premised on an all-for-one-and-one-for-all approach to defeating the pandemic”, adding that “this would have led to the best outcomes for everyone and was our best hope for ending the pandemic quickly. But we also know from experience that the world doesn’t really work this way.”
Everyone knew that rich countries would enter into bilateral vaccine deals, Yamey said. But it was hoped that they would also buy into COVAX as insurance in case some vaccine candidates did not prove successful. Most of them did not. In the end, “three dozen countries bypassed COVAX and made huge deals directly with manufacturers. They were very lucky that the vaccines worked out. And since they cleared the shelves, there were not enough doses left for COVAX”, he said.
Sweeteners
As rich countries busily signed bilateral agreements with individual vaccine manufacturers and the interest in engaging with COVAX faded, Gavi gave up on the original idealistic approach and made two key concessions that would sweeten the deal for self-financing participants.
Initially, all countries were to receive equal treatment by COVAX. They would have access to vaccines at the same time and participants would be allowed to purchase enough doses to cover 20% of their populations. Moreover, participants were to be “product agnostic”, in the sense that COVAX would decide on the products and allocation of volume of doses.
Breaking with the principle of equal treatment, Gavi created a second category of purchase options for self-financing countries, called the Optional Purchase Arrangement, which gave buyers the possibility to opt in or out of certain products; basically, giving them more choice about which vaccines they would receive. That is, if a country was offered vaccine A but did not want it, it would not be obliged to take it, and could instead save its options for purchase of another product. “The trade-off for these participants, who will have greater choice, is that they will be required to pay a higher proportion of the total cost per dose upfront”, states a Gavi explainer. This, Kate Elder at Médecins Sans Frontières said, was done in response to pressure from the UK. “Gavi bent over backwards and let the UK basically dictate another option for joining COVAX”, she said.
A second concession made by Gavi to potential self-financing countries—but not open to lower-income countries—was an increase in the volume of vaccine they were permitted to purchase. While countries eligible for the AMC window were intended to receive vaccines to cover up to 20% of their populations, the ceiling for self-financing countries was raised to 50%.
Elder points to the blatant inequity built into this arrangement. She said Gavi lost valuable time last year coaxing rich countries to join COVAX. “The theory that you would get every country to buy into this global procurement mechanism seemed very naive”, she said. “COVAX sacrificed speed to convince governments to join the initiative, when clearly [those governments] were going to take other steps to secure vaccines. I think acquiescing to the ‘suggestions’ of a small group of HICs led to the overall weakening of COVAX, because it introduced a lot of uncertainty in the mechanism itself…The delayed timeline also led to a delay in the fundraising.”
The report of the Independent Panel for Pandemic Preparedness and Response also pointed to the harm caused by the slow mobilisation of resources for COVAX: “Had COVAX had sufficient and readily available early funding it would have been better able to secure enough immediate supply to meet its aims”, it states.
“If we had secured financing earlier, then we could have locked in doses earlier, as opposed to the second half of this year when COVAX’s volume will start ramping up”, Gavi’s spokesperson told The Lancet.
Yamey said the concessions that were offered to wealthier nations “speak to the effort to incentivise rich countries to join COVAX. For future pandemics, we have to figure out how to overcome this problem. I think that we need a compulsory mechanism where every nation participates now. Otherwise, you need to find stronger incentives… This is a difficult nut to crack.”
Gavi’s spokesperson said that “COVAX was set up as a multilateral mechanism, and active engagement and collaboration has been its driving force since June, 2020, when it was launched. COVAX’s governance structure is designed so that lower-income economies and self-financing countries all have a say in the strategic direction of the Facility.”
Gavi’s predicament
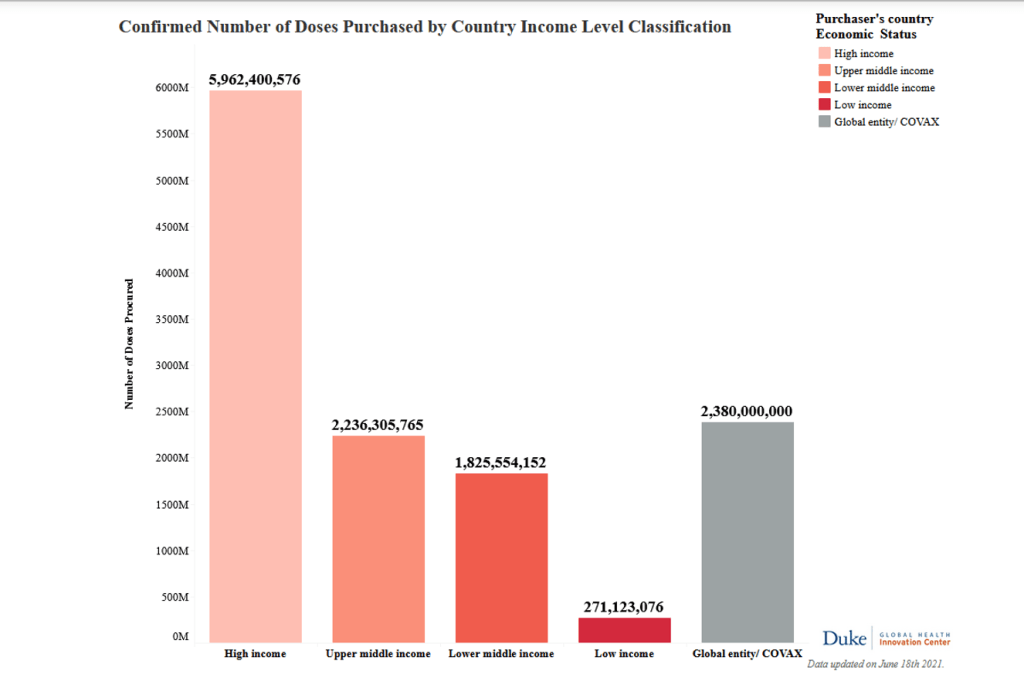
The failure to entice wealthy countries to join COVAX in large numbers has left the managers of the facility in an awkward situation. On one hand, not enough self-financing participants joined COVAX to give it the massive buying power that was hoped. On the other, even though COVAX is desperately short of vaccine, the facility is now contractually obliged to reserve one in five doses for a few rich countries. As of late May, COVAX had supplied about 80 million doses to LMICs; 22 million doses had gone to HICs.
While the access inequities have widened, Gavi has had to justify sending vaccines to countries that have already vaccinated a large portion of their populations at the same time as deliveries to the very poorest countries have barely begun. This uncomfortable predicament is palpable in Gavi’s messaging about COVAX, which now rarely, if ever, mentions the self-financing part of the facility.
Although Gavi has produced numerous press releases about deliveries of vaccines to LMICs, starting with the shipment of 600 000 doses of the Oxford University–AstraZeneca vaccine to Ghana on Feb 24, 2021, there was no announcement when Canada was allocated 1·62 million doses of the same vaccine earlier that month, and no fanfare when 500 000 doses of the Pfizer–BioNTech vaccine were assigned by COVAX to the UK in April.
Oxfam criticised Canada for the delivery, accusing the Government of taking doses from the poor when it has signed bilateral deals with manufacturers for enough vaccine to cover four times the country’s population. “Canada should not be taking the COVAX vaccine from poor nations to alleviate political pressures at home”, Diana Sarosi at Oxfam Canada said. However, strictly speaking, Canada was merely following through on the terms of its agreement with COVAX. Public services and procurement minister Anita Anand said as much when she defended the move in a comment to CBC. Canada is “entitled under our agreement with COVAX to draw down on the commitment that we made with them back in the summer”, she said.
Similarly, responding to a question in parliament in March, 2021, the UK under-secretary for health and social care Nadhim Zahawi explained that COVAX had enabled “high- and upper-middle income countries to pool investments in potential vaccine candidates”. Both the Canadian and British governments pointed to their generous donations to the AMC part of COVAX, which is dedicated to supplying vaccine to poor countries.
The self-financing window of COVAX has also disappeared from the budget of the Access to COVID-19 Tools Accelerator (ACT-A), of which the COVAX facility is a part. The first iteration of the budget in September, 2020, had a total financial frame of US$38 billion. By March, 2021, the ask had dropped to US$33 billion. Norway’s Global Health Ambassador John-Arne Røttingen, a Gavi board member, explained why this happened: “The fundraising necessary for the COVAX facility and the fundraising for ACT-A when it comes to vaccines is only relevant for the AMC window and not for the self-financing countries, since that is where we mobilise collective finance. That is why we took that out of the budgeting.”
COVAX needs more doses
COVAX now aims to roll out 2·3 billion doses of COVID-19 vaccines worldwide by early 2022. According to the latest COVAX global supply forecast, dated April 7, 2021, 485 million doses of these are earmarked for self-financing countries, while 1·8 billion doses will go to the 92 lower-income countries, with at least 1·3 billion of those doses available at no cost to their governments.
This volume of doses is not assured: “If the forecast comes to pass—and that is a big if, with uncertainties around capacity, funding, and country readiness—this means that COVAX should be able to reach at least 27 per cent of the population of lower-income countries across the world in 2021, well above the 20 per cent target it set upon its inception”, Gavi states. Many have questioned the original COVAX target of 20%. Even 27% coverage will leave countries that are reliant on COVAX well short of the volume of vaccines they need to achieve herd immunity.
“I have always thought that the 20% target was unfair from the start. No high-income country would tolerate vaccinating only 20% of its population by the end of this year”, said Lawrence Gostin at Georgetown University. Comparing the 20% vaccination target for LMICs with the 50% coverage that was offered to HICs through the self-financing window, he said: “We can’t have double standards.”
WHO estimates that the world needs at least 11 billion doses of vaccine to stamp out the pandemic, and the European Commission has warned that new variants of SARS-CoV-2 that are more transmissible and more deadly could “[push] the demand far beyond the 11 billion doses originally estimated”.
Underlining the scale of the challenge, The People’s Vaccine Alliance—a coalition of organisations including Oxfam and Amnesty International, health experts, and world leaders, that has lobbied for a waiver on COVID-19-related patents—estimates that, at the current rate, low-income countries could take 57 years to fully vaccinate their populations, whereas G7 countries might reach that milestone in the next 6 months.
Against this backdrop, and recognising that wealthy countries have ordered more vaccine doses than they need, dose sharing has emerged as a way to radically increase COVAX’s access to doses. As of late May, 2021, HICs had promised to share 200 million doses with COVAX. Many, including the International Monetary Fund and the Bill & Melinda Gates Foundation, argued that 1 billion doses can and should be shared this year. The Independent Panel recommended that at least 1 billion vaccine doses be shared by no later than Sept 1, 2021, and more than 2 billion doses by mid-2022.
There were therefore huge expectations for last week’s G7 summit, both for dose sharing and for more funding of tools other than vaccines provided through ACT-A, such as protective equipment for health workers, tests, and medical oxygen, the demand for which has increased five times compared with pre-pandemic levels. The group of wealthy countries did not commit new funding for ACT-A, and ended up pledging only 870 million doses over the next year “primarily to COVAX”.
It is uncertain how quickly this will result in vaccines being administered. The Biden administration’s decision to donate 500 million doses of the Pfizer–BioNTech COVID-19 vaccine has caused concern because many countries lack the necessary cold-chain infrastructure. Additionally, while the UK has promised to give away 100 million doses “within the next year”, most of these will not be released until 2022.
The G7 pledge was roundly criticised by Amnesty International, which called it “a drop in the ocean [that] wouldn’t come close to covering the population of India, let alone vaccinating the world’s population” and said that “G7 leaders have opted for more of the same paltry half measures and insufficient gestures”.
Moreover, the G7 communique commits to deliver only half of the 870 million doses by the end of the year—ie, around 450 million doses. Since COVAX is contractually obliged to deliver 485 million of the 2·3 billion doses to self-financing (high-income) countries this year, the G7 dose-sharing pledge does not quite match the volume of vaccines that rich countries are set to take out of COVAX for their own domestic programmes.
The COVAX model
Taylor likens the process of designing COVAX to building a car while you are driving it. In early 2020, the shape and timeline of the pandemic were unknown, the symptoms of the disease were not well understood, and no-one knew how long it would take for one or more vaccines to be developed.
The original notion of a global vaccine hub more or less collapsed, and COVAX ended up using a traditional aid-financed approach, which has left lower-income countries wholly at the mercy of wealthy nations and profit-driven companies.
“It is still this model of seeing how much money you can bring in and then seeing what you can negotiate with industry based on that money”, said Elder. “The promise of COVAX from the beginning that it would be the most attractive buyer for industry because it represented the ‘global need’ obviously did not pan out.” For any future iterations of COVAX, Taylor has argued that since national leaders have a responsibility to protect their own populations, vaccine nationalism is inevitable and this should be integrated into the design from the start.
Several global health experts point to the failure to recognise supply constraints as a major obstacle to global vaccination and emphasise diversifying and scaling up manufacturing from the beginning. This lack of recognition was a serious flaw in the COVAX design, said Gostin. “Supply shortages should have been anticipated and ramping up supplies should have been baked into the design of COVAX from the start.”
The Gavi spokesperson said many lessons have been learned along the way. “We need to start expanding vaccine manufacturing—in particular in the Global South—now, if we want to respond better during the next pandemic. Other innovations COVAX has developed, such as its universal no-fault compensation scheme, have made it easier for manufacturers to provide vaccines to lower-income countries and will help future responses too.”
The UN secretary general has called for a global task force on vaccination that would bring together ACT-A partners with the multilateral system and would be able to “deal with pharmaceutical companies”. Guterres implies an approach that is much tougher on industry than ACT-A and COVAX’s voluntary, partnership-based approach.
“Guterres is right”, said Gostin. “There can be no solution to the global vaccine crisis without governments placing pressure on big pharma, including waiving intellectual property rights and technology transfer. It is literally impossible to ramp up vaccine supplies unless we have more manufacturing hubs, including in lower-income countries.”
Recognising the shortcomings of COVAX is likely to be important far beyond the current pandemic. The COVAX approach is already being touted as a possible model for dealing with future pandemics and other global crises, such as climate change. In a report on development cooperation during 2020, Susanna Moorehead, chair of the OECD Development Assistance Committee, writes: “Lessons from the COVAX facility can inform the design of co-ordinated platforms to promote global public goods, including those to mitigate the impact of climate change.” In a similar vein, the Independent Panel proposes that ACT-A, including COVAX, could serve as a model for a permanent mechanism that would transform HIC-dominated systems to a global, inclusive approach. “ACT-A provides a valuable model. Lessons drawn from both its strengths and weaknesses should guide the establishment of a permanent platform which can stand in readiness for any future pandemic.”
Yamey warns that the success of COVAX and ACT-A is far from assured. He and colleagues have argued that monopolies on knowledge and production that benefit a handful of companies have locked lower-income countries out, while rich countries have used their power to put their populations ahead of the most vulnerable globally. “I have a dog in the race—I would like to see the COVAX thrive [but] unless we address these structural issues, what advantage is there to making ACT-A permanent?”