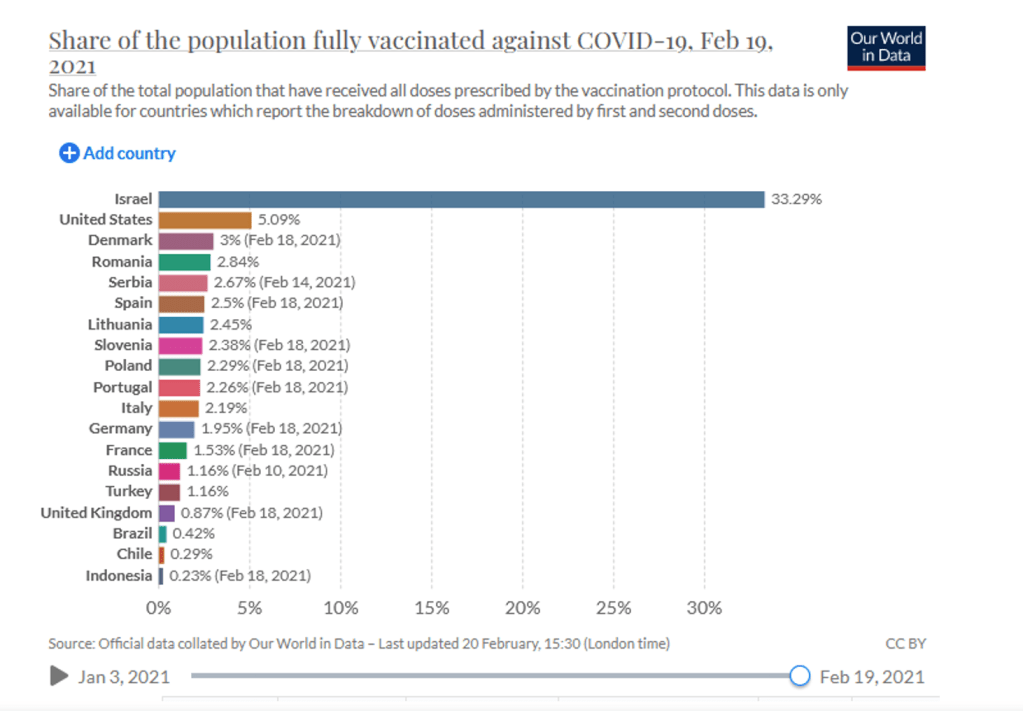
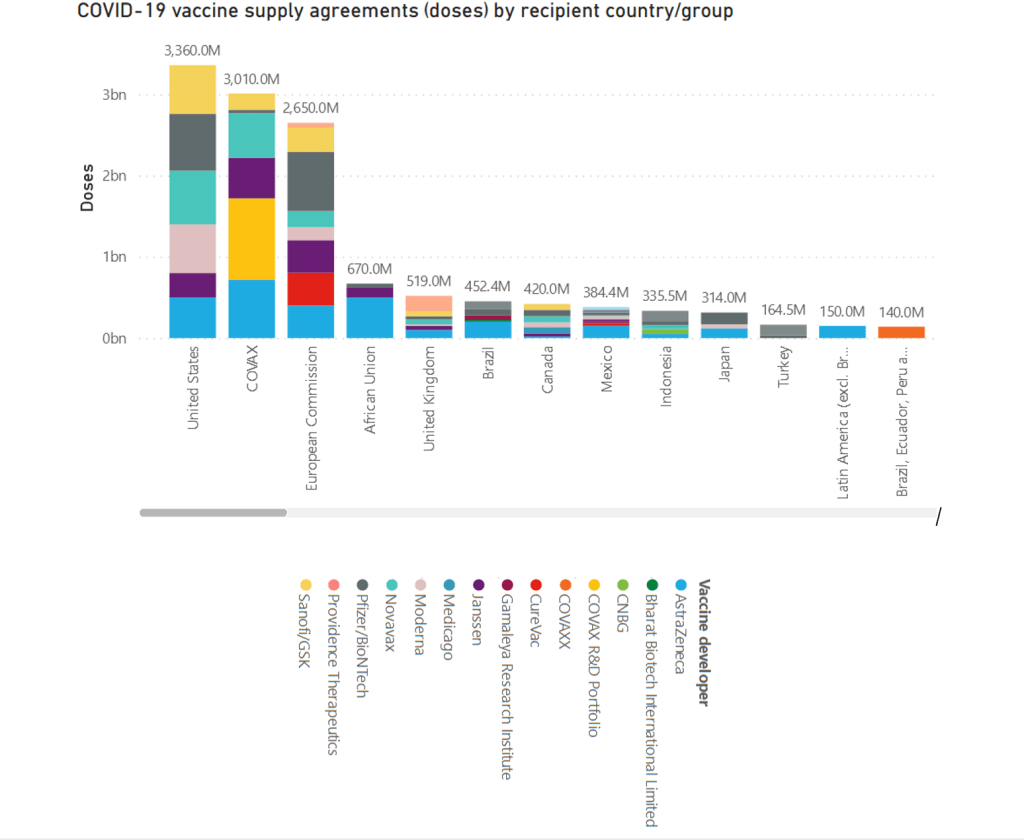
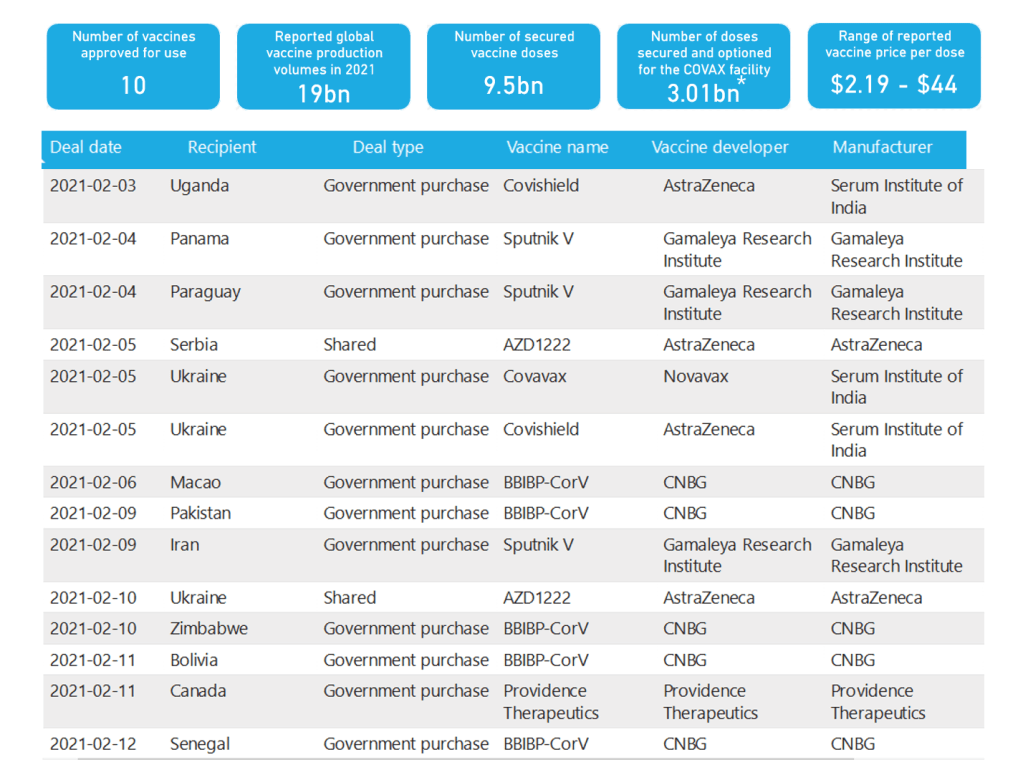
Organization Announcements
Paul G. Allen Frontiers Group [to 20 Feb 2021]
https://alleninstitute.org/what-we-do/frontiers-group/news-press/
News
No new digest content identified.
BARDA – U.S. Department of HHS [to 20 Feb 2021]
https://www.phe.gov/about/barda/Pages/default.aspx
BARDA News
No new digest content identified.
BMGF – Gates Foundation [to 20 Feb 2021]
http://www.gatesfoundation.org/Media-Center/Press-Releases
Press Releases and Statements
No new digest content identified.
Bill & Melinda Gates Medical Research Institute [to 20 Feb 2021]
https://www.gatesmri.org/
The Bill & Melinda Gates Medical Research Institute is a non-profit biotech organization. Our mission is to develop products to fight malaria, tuberculosis, and diarrheal diseases—three major causes of mortality, poverty, and inequality in developing countries. The world has unprecedented scientific tools at its disposal; now is the time to use them to save the lives of the world’s poorest people
No new digest content identified.
CARB-X [to 20 Feb 2021]
https://carb-x.org/
News
No new digest content identified.
Center for Vaccine Ethics and Policy – GE2P2 Global Foundation [to 20 Feb 2021]
https://centerforvaccineethicsandpolicy.net/
News/Analysis/Statements
Past weekly editions of Vaccines and Global Health: The Week in Review are available here.
CEPI – Coalition for Epidemic Preparedness Innovations [to 20 Feb 2021]
http://cepi.net/
Latest News
New infectious disease R&D grant opportunity launches for Canadian researchers
18 Feb 2021
By Jodie Rogers
CEPI and the Canadian Institutes of Health Research Institute of Infection and Immunity (CIHR-III), one of the 13 virtual institutes of Canada’s federal funding agency for health research, CIHR, have today launched the CIHR-CEPI Leadership Award for Excellence in Vaccine Research for Infectious Diseases of Epidemic Potential funding opportunity.
The award is a new two-year grant funding opportunity for up to six independent mid-career researchers in Canada to pursue projects—co-developed alongside CEPI—to expedite the development of vaccines against emerging infectious diseases, including COVID-19. The Request for Applications is now available on the CIHR-III website in English and in French...
COVAX Statement on WHO Emergency Use Listing for AstraZeneca/Oxford COVID-19 Vaccine
Geneva / New York / Oslo – 16 February 2021 The Coalition for Epidemic Preparedness Innovations (CEPI), Gavi, the Vaccine Alliance (Gavi), and the World Health Organization (WHO), as co-leads of the COVAX initiative for equitable global access to COVID-19 vaccines, alongside key delivery partner UNICEF, are pleased to welcome the news that two versions of the AstraZeneca/Oxford COVID-19 vaccine have been given WHO Emergency Use Listing (EUL). Yesterday’s announcement means that two versions of the AstraZeneca/Oxford vaccine, produced by AstraZeneca-SK Bioscience (AZ-SKBio) and the Serum Institute of India (AZ-SII), are now available for global rollout through the COVAX Facility…
DARPA – Defense Advanced Research Projects Agency [to 20 Feb 2021
https://www.darpa.mil/
News
No new digest content identified.
Duke Global Health Innovation Center [to 20 Feb 2021]
https://dukeghic.org/
Launch and Scale Speedometer
No new digest content identified.
EDCTP [to 20 Feb 2021]
http://www.edctp.org/
The European & Developing Countries Clinical Trials Partnership (EDCTP) aims to accelerate the development of new or improved drugs, vaccines, microbicides and diagnostics against HIV/AIDS, tuberculosis and malaria as well as other poverty-related and neglected infectious diseases in sub-Saharan Africa, with a focus on phase II and III clinical trials
No new digest content identified.
Emory Vaccine Center [to 20 Feb 2021]
http://www.vaccines.emory.edu/
Vaccine Center News
No new digest content identified.
European Commission [to 20 Feb 2021]
http://europa.eu/rapid/search-result.htm?query=18&locale=en&page=1
Latest
Press release 19 February 2021
G7: EU to support COVID-19 vaccination strategies and capacity in Africa
The President of the European Commission, Ursula von der Leyen, has announced today €100 million in humanitarian assistance to support the rollout of vaccination campaigns in Africa, which are spearheaded by the Africa Centres for Disease Control and Prevention (Africa CDC).
Press release 19 February 2021
New EU project to support readiness for vaccination efforts and resilient health systems in the Western Balkans
The European Union in partnership with the World Health Organisation (WHO) has launched a new regional project worth over €7 million to support safe and effective vaccination of the populations across the Western Balkans.
Press release 19 February 2021
EU doubles contribution to COVAX to €1 billion to ensure safe and effective vaccines for low and middle-income countries
The European Union has announced today an additional €500 million for the COVAX Facility, doubling its contribution to date for the global initiative that is leading efforts to secure fair and equitable access to safe and effective COVID-19 vaccines in low and middle-income countries.
News 17 February 2021
Von der Leyen announces the start of HERA Incubator to anticipate the threat of coronavirus variants
The HERA Incubator will bring together science, industry and public authorities, and leverage all available resources to enable Europe to respond to this challenge.
Press release 17 February 2021
Coronavirus: Commission approves second contract with Moderna to ensure up to additional 300 million doses
Today, the European Commission approved a second contract with the pharmaceutical company Moderna, which provides for an additional purchase of 300 million doses (150 million in 2021 and an option to purchase an additional 150 million in 2022) on behalf of all EU Member States.
European Medicines Agency [to 20 Feb 2021]
http://www.ema.europa.eu/ema/
News & Press Releases
News: EMA receives application for conditional marketing authorisation of COVID-19 Vaccine Janssen
Last updated: 16/02/2021
EMA has received an application for conditional marketing authorisation (CMA) for a COVID-19 vaccine developed by Janssen-Cilag International N.V.
EMA’s human medicines committee (CHMP) will assess the vaccine, known as COVID-19 Vaccine Janssen, under an accelerated timetable. The Committee could issue an opinion by the middle of March 2021, provided the company’s data on the vaccine’s efficacy, safety and quality are sufficiently comprehensive and robust.
Such a short time for evaluation is only possible because EMA has already reviewed some data during a rolling review…
European Vaccine Initiative [to 20 Feb 2021]
http://www.euvaccine.eu/
Latest News
EVI
17 Feb 2021
EVI to lead Immune Monitoring in pan-European vaccine trial network, VACCELERATE
EVI to lead Immune Monitoring in pan-European vaccine trial network, VACCELERATE, as part of €150 million EU investment in research to counter coronavirus variants.
The European Commission announced today a €150 million investment for research to counter coronavirus variants. As part this “European Health Emergency Preparedness and Response Authority (HERA) Incubator” programme, Horizon 2020 funding will support the rapid establishment of a new EU-wide vaccine trial network called VACCELERATE. The grant agreement was signed today between representatives of the VACCELERATE consortium and the European Commission.
VACCELERATE will receive a €12 million grant and involves 26 partners, including the European Vaccine Initiative (EVI), in 16 Member States (AT, BE, CY, DE, DK, EL, ES, FR, IE, IT, LT, NL, PL, PT, SE, SK) and 5 associated countries (CH, IL, NO, RS, TR). VACCELERATE network, led by University Hospital Cologne, will act as a single entry point for vaccine developers, including SMEs, who are looking for European infrastructure to carry out vaccine trials. The consortium will work closely with the European Medicines Agency (EMA) to enable clinical trials for COVID-19 vaccines and prepare Europe for other emerging infectious diseases in the future.
EVI will have a key role in the project by leading the immune monitoring activities, which will focus on compiling, standardizing and prioritizing a comprehensive catalogue of immunological and genetic assays for COVID-19 vaccines. This will involve close collaboration with consortium partners as well as with other initiatives and networks that strive to standardise assessment of COVID-19 vaccines…
FDA [to 20 Feb 2021]
https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/default.htm
Press Announcements /Selected Details
:: February 19, 2021 – Coronavirus (COVID-19) Update: February 19, 2021
:: February 18, 2021 – COVID-19 Update: USDA, FDA Underscore Current Epidemiologic and Scientific Information Indicating No Transmission of COVID-19 Through Food or Food Packaging
:: February 16, 2021 – Coronavirus (COVID-19) Update: February 16, 2021
FDA – COVID-19 Vaccines [to 20 Feb 2021]
www.fda.gov/covid19vaccines
News and Updates; Upcoming Events
No new digest content identified.
Fondation Merieux [to 20 Feb 2021]
http://www.fondation-merieux.org/
News, Events
Mérieux Foundation co-organized event
MERACON: Returning to rabies elimination in 2021 webinar
February 26, 2021 – 9:00am -12:00pm (CET)
Context
The COVID-19 pandemic has hampered global travel and has placed a strain on public health systems globally. Because of this, many rabies activities were postponed or cancelled in 2020. Similarly, the planned in-person regional MERACON workshop that was initially scheduled for mid-2020 was postponed to 2021. As a means to continue remote support, foster collaboration and continue to drive progress towards rabies elimination in the MERACON countries, the MERACON steering committee has scheduled a digital meeting in webinar format. This webinar will act as a precursor to a more detailed workshop later in the year which we hope to host in-person (COVID-19 restrictions permitting).
Gavi [to 20 Feb 2021]
https://www.gavi.org/
News Releases
G7 backs Gavi’s COVAX Advance Market Commitment to boost COVID-19 vaccines in world’s poorest countries
19 February 2021
Gavi signs memorandum of understanding with Novavax on behalf of COVAX Facility
18 February 2021
COVAX Statement on WHO Emergency Use Listing for AstraZeneca/Oxford COVID-19 Vaccine
16 February 2021
[See COVID above for detail on these announcements]
GHIT Fund [to 20 Feb 2021]
https://www.ghitfund.org/newsroom/press
GHIT was set up in 212 with the aim of developing new tools to tackle infectious diseases that
Press Releases
No new digest content identified.
Global Fund [to 20 Feb 2021]
https://www.theglobalfund.org/en/news/
News & Stories
No new digest content identified.
Global Research Collaboration for Infectious Disease Preparedness [GloPID-R] [to 20 Feb 2021]
https://www.glopid-r.org/news/
News
No new digest content identified.
Hilleman Laboratories [to 20 Feb 2021]
http://www.hillemanlabs.org/
No new digest content identified.
Human Vaccines Project [to 20 Feb 2021]
http://www.humanvaccinesproject.org/media/press-releases/
Press Releases
Science, 19 FEBRUARY 2021 • VOL 371 ISSUE 6531
Editorial: A univer sal coronavirus vaccine
Wayne C . Koffi, chief executive officer of the Human Vaccines Project; Seth Berkley, chief executive officer of Gavi, The Vaccine Alliance, Geneva, Switzerland.
[See COVID above for detail]
IAVI [to 20 Feb 2021]
https://www.iavi.org/newsroom
PRESS RELEASES/FEATURES
No new digest content identified.
International Coalition of Medicines Regulatory Authorities [ICMRA]
http://www.icmra.info/drupal/en/news
Selected Statements, Press Releases, Research
ICMRA COVID-19 Virus Variants Workshop
10 February 2021
Co-chairs: Marion Gruber (FDA, US) and Marco Cavaleri (EMA, EU)
[See COVID above for detail]
International Generic and Biosimilar Medicines Association [IGBA]
https://www.igbamedicines.org/
*News
Press Releases/Announcements
No new digest content identified.
IFFIm
http://www.iffim.org/
Press Releases/Announcements
No new digest content identified.
IFRC [to 20 Feb 2021]
http://media.ifrc.org/ifrc/news/press-releases/
Selected Press Releases, Announcements
Kazakhstan
Kazakhstan: IFRC and Red Crescent launch bot to counter COVID misinformation
A social media chatbot has been launched in an innovative bid to share accurate, trusted information in a country with one of the world’s highest rates of vaccine hesitancy
19 February 2021
UN Security Council session on COVID-19: IFRC warns of combined dangers of mistrust and vaccine inequity
17 February 2021
[See COVID above for detail]
Africa, Guinea
Ebola outbreak in Guinea: Red Cross calls for a response that is “faster than the virus”
A network of more than 700 trained Red Cross volunteers has been activated as part of a first wave of response to the new Ebola outbreak in the rural community of Gouéké in Guinea’s N’Zerekore prefecture.
15 February 2021
Institut Pasteur [to 20 Feb 2021]
https://www.pasteur.fr/en/press-area
Press release 18.02.2021
Improving immunotherapies for blood cancers: real-time exploration in the tumor
Monoclonal antibodies are part of the therapeutic arsenal for eliminating cancer cells. Some make use of the immune…
IRC International Rescue Committee [to 20 Feb 2021]
http://www.rescue.org/press-release-index
Media highlights [Selected]
Press Release
IRC joins #FirstRespondersFirst initiative to help humanitarian staff manage the psychological toll of responding to COVID-19 on the frontlines
February 18, 2021
Press Release
As new Ebola outbreaks emerge in the DRC and Guinea, the IRC calls for swift action and funding for frontline aid agencies to stop the spread
February 15, 2021
IVAC [to 20 Feb 2021]
https://www.jhsph.edu/research/centers-and-institutes/ivac/index.html
Updates; Events
No new digest content identified.
IVI [to 20 Feb 2021]
http://www.ivi.int/
Selected IVI News, Announcements, Events
No new digest content identified.
JEE Alliance [to 20 Feb 2021]
https://www.jeealliance.org/
Selected News and Events
No new digest content identified.
Johns Hopkins Center for Health Security [to 20 Feb 2021]
https://www.centerforhealthsecurity.org/news/center-news/
Center News
Health Security Releases Special Feature on Infodemics
February 19, 2021
New Report: Staying Ahead of the Variants: Policy Recommendations to Identify and Manage Current and Future Variants of Concern
February 16, 2021
The Johns Hopkins Center for Health Security released a new report on the status of SARS-CoV-2 surveillance, sequencing, and variant characterization and actions the U.S. government should take to increase capacity to respond to new virus variants.
The 3 new concerning variants of SARS-CoV-2 could make the virus spread more easily or make therapeutics and vaccines for COVID-19 less effective. And as the pandemic unfolds, more variants will emerge and spread…
PDF: https://www.centerforhealthsecurity.org/our-work/publications/staying-ahead-of-the-variants
MSF/Médecins Sans Frontières [to 20 Feb 2021]
http://www.msf.org/
Latest [Selected Announcements]
Guinea
Five questions on the Ebola outbreak in Guinea
Interview 19 Feb 2021
Coronavirus COVID-19 pandemic
Southern Africa needs the right COVID-19 vaccines, at the right price – right now
Press Release 18 Feb 2021
Myanmar
MSF concerned for welfare of healthcare workers and people in Myanmar
Statement 17 Feb 2021
Iraq
Severe COVID-19 patients in Iraq “were almost sure to die”
Project Update 16 Feb 2021
National Vaccine Program Office – U.S. HHS [to 20 Feb 2021]
https://www.hhs.gov/vaccines/about/index.html
Upcoming Meetings/Latest Updates
NVAC Meeting – February 4-5, 2021
Agenda
NIH [to 20 Feb 2021]
http://www.nih.gov/news-events/news-releases
News Releases
To end HIV epidemic, we must address health disparities
February 19, 2021 — Expert report cites unequal progress in Southern U.S. and among marginalized groups.
NIH funds study to evaluate remdesivir for COVID-19 in pregnancy
February 17, 2021 — The study will be conducted at 17 sites in the continental United States and Puerto Rico.
PATH [to 20 Feb 2021]
https://www.path.org/media-center/
Press Releases
No new digest content identified.
Sabin Vaccine Institute [to 20 Feb 2021]
http://www.sabin.org/updates/pressreleases
Statements and Press Releases
No new digest content identified.
UNAIDS [to 20 Feb 2021]
http://www.unaids.org/en
Selected Press Releases/Reports/Statements
15 February 2021
Félix Tshisekedi, President of the Democratic Republic of the Congo and 2021 African Union Chair, calls on his peers to learn from HIV and strengthen health systems
15 February 2021
Tuberculosis testing gap among people living with HIV is narrowing
UNHCR Office of the United Nations High Commissioner for Refugees [to 20 Feb 2021]
http://www.unhcr.org/en-us/media-centre.htmlS
Selected Announcements
Humanitarian agencies seek US$222 million to support Burundian refugees
16 February 2021
UNHCR alarmed at armed atrocities in eastern DR Congo
This is a summary of what was said by UNHCR spokesperson Babar Baloch – to whom quoted text may be attributed – at today’s press briefing at the Palais des Nations in Geneva.
16 February 2021
UNICEF [to 20 Feb 2021]
https://www.unicef.org/media/press-releases
Selected Press releases, Statements
No new digest content identified.
Unitaid [to 20 Feb 2021]
https://unitaid.org/
Featured News
19 February 2021
Germany contributes €20 million to Unitaid’s vital work on COVID-19
…The funding from Germany will contribute to the realisation of key aspects of the new ACT-Accelerator Therapeutics Partnership strategy (co-led by Unitaid and Wellcome), including advancing research on new treatments for COVID-19, ensuring affordable supplies and country preparedness so that low- and middle-income countries are able to use treatments effectively, further allowing for the procurement of proven treatments, as and when they become available….
19 February 2021
Unitaid welcomes the appointment of Dr. Ngozi Okonjo-Iweala to lead WTO
Vaccination Acceptance Research Network (VARN) [to 20 Feb 2021]
https://vaccineacceptance.org/news.html#header1-2r
Announcements
No new digest content identified.
Vaccine Confidence Project [to 20 Feb 2021]
http://www.vaccineconfidence.org/
News, Research and Reports
No new digest content identified.
Vaccine Education Center – Children’s Hospital of Philadelphia [to 20 Feb 2021]
http://www.chop.edu/centers-programs/vaccine-education-center
News
No new digest content identified.
Wellcome Trust [to 20 Feb 2021]
https://wellcome.ac.uk/news
News
Explainer
What are human infection studies and why do we need them?
Human infection studies (also known as human challenge trials and controlled human infection models) have the power to rapidly accelerate the development of much-needed vaccines and treatments, including for Covid-19.
18 February 2021
Opinion
We’re backing the AMR Action Fund – this is what it means for antibiotic innovation
Wellcome joins a new collaboration of private and philanthropic partners in the search for new antibiotics to treat drug-resistant infections. Tim Jinks explains why this is important and why the next move needs to come from governments.
17 February 2021
The Wistar Institute [to 20 Feb 2021]
https://www.wistar.org/news/press-releases
Press Releases
No new digest content identified.
WFPHA: World Federation of Public Health Associations [to 20 Feb 2021]
https://www.wfpha.org/
Latest News
No new digest content identified.
World Bank [to 20 Feb 2021]
http://www.worldbank.org/en/news/all
Selected News, Announcements
How COVID-19 is Affecting Companies Around the World
Date: February 17, 2021 Type: Infographic
Almost a year into the pandemic, nearly every business in the world has been affected by COVID-19, but performance has varied widely, even within countries and industries. Data collected through the World Bank firm surveys offer some glimpses into why, and how this may be relevant for policy.
Statement of Mr. Ferid Belhaj, Vice President of the Middle East and North Africa Region, The World Bank: Launch of the National COVID-19 Vaccine Campaign in Lebanon
As prepared for delivery
Date: February 14, 2021 Type: Statement
World Organisation for Animal Health (OIE) [to 20 Feb 2021]
https://www.oie.int/en/for-the-media/press-releases/2021/
Press Releases
No new digest content identified.
::::::
ARM [Alliance for Regenerative Medicine] [to 20 Feb 2021]
Press Releases – Alliance for Regenerative Medicine (alliancerm.org)
Press Releases
No new digest content identified.
BIO [to 20 Feb 2021]
https://www.bio.org/press-releases
Press Releases
Fresh Perspective for BIO Operations, Sustainability
BIO taps MN biotech leader, Shaye Mandle, as new Chief Operating Officer
February 16, 2021
Washington, D.C. – As the Biotechnology Innovation Organization (BIO) continues to restructure for a stronger and reinvigorated future, the organization announced new leadership for operations and sustainability. Shaye Mandle was named Chief Operating Officer (COO), effective March 1, 2021…
DCVMN – Developing Country Vaccine Manufacturers Network [to 20 Feb 2021]
http://www.dcvmn.org/
News; Upcoming events
16 February 2021
WHO lists two COVID-19 vaccines from DCVMN members for emergency use
ICBA – International Council of Biotechnology Associations [to 20 Feb 2021]
https://internationalbiotech.org/news/
News
No new digest content identified.
IFPMA [to 20 Feb 2021]
http://www.ifpma.org/resources/news-releases/
Selected Press Releases, Statements, Publications
The AMR Action Fund announces appointment of CEO, Henry B. Skinner, PhD
18 February 2021
Dr. Skinner will help ensure the AMR Action Fund achieves its mission to bring two to four new antibiotics to patients by the end of the decade and facilitate needed long-term policy solutions
FEBRUARY 18, 2021, BOSTON, MA — Today, the AMR Action Fund announced the appointment of its first Chief Executive Officer (CEO), Henry B. Skinner, PhD. The AMR Action Fund (www.AMRactionfund.com) is a ground-breaking partnership that was launched in July 2020 by more than 20 leading pharmaceutical companies. It aims to invest over US$1 billion to strengthen and accelerate clinical research of innovative new antibiotics that are addressing the most resistant bacteria and life-threatening infections, as well as provide technical support to biotech companies developing assets in this field. The AMR Action Fund is the largest collaborative venture ever created to address antimicrobial resistance (AMR)…
The AMR Action Fund announces its first non-industry investments, raising an additional US$140 million toward
18 February 2021
The AMR Action Fund, launched in July 2020, aims to bring 2 to 4 new antibiotics to patients by the end of the decade and facilitate needed long-term solutions
FEBRUARY 18, 2021, BOSTON — Today, the AMR Action Fund announced its first initial close with non-pharmaceutical industry investments of more than US$140 million from the Boehringer Ingelheim Foundation, the European Investment Bank (supported by the European Commission), and the Wellcome Trust. This adds to the initial investment from more than 20 leading biopharmaceutical companies that established the Fund as announced in July 2020. With this significant financial commitment from global foundations and development banks, the AMR Action Fund becomes the world’s largest public-private partnership supporting the development of new antibiotics. It demonstrates the type of commitment and collaboration across sectors that is urgently needed to address the global threat of antibiotic-resistant infections – also called antimicrobial resistance, or AMR…
Global Principles on Incentivizing Antibiotic R&D
IFPMA Policy Position :: 15 February 2021 :: 11 pages
PDF: https://www.ifpma.org/wp-content/uploads/2021/02/IFPMA-Global-Principles-on-Incentivizing-Antibiotic-RD.pdf
[Excerpt]
…We therefore call on all countries to take steps now to deliver a clear implementation roadmap by the end of 2021, make meaningful progress in implementation by 2023, and ensure full and effective implementation by 2025 at the latest of:
[1] New economic incentives, giving confidence to the private sector to invest in R&D at the level needed to create a robust antibiotic pipeline.
[2] Bespoke valuation of antibiotics, assessing and recognizing the full value antibiotics deliver to society and correcting their current under-valuation.
[3] Reimbursement reforms, to maintain availability of antibiotics on the market and to enable patient access to the most appropriate antibiotic to treat or prevent their infection.
While the solutions to these challenges will look different in different countries – there is no ‘one size fits all’ – this supportive policy framework is necessary to drive long-term investments in innovative antibiotics, throughout the discovery, development, and product lifecycle. In this paper, we propose a set of principles and models to establish such a framework, and we make an urgent call to Governments to drive these reforms to implementation, thereby delivering on recent G7 and G20 commitments..
PhRMA [to 20 Feb 2021]
http://www.phrma.org/
Selected Press Releases, Statements
The latest: What they are saying: Intellectual property protections vital to COVID-19 research, development and manufacturing
February 12, 2021
Strong and reliable IP protections – including patents – have supported America’s robust innovation ecosystem by promoting discovery, development, affordability and access to new treatments and cures. As our industry continues to expand vaccine production and deliver medicines to patients in need, reliable IP protections have been critical in supporting multiple research and development and manufacturing ramp-ups on COVID-19 vaccines and therapeutics. Innovators need strong and reliable IP protections to research, develop and manufacture new therapeutics and vaccines that will improve patients’ lives during the current pandemic and beyond…
Blog Post