Media/Policy Watch
This watch section is intended to alert readers to substantive news, analysis and opinion from the general media and selected think tanks and similar organizations on vaccines, immunization, global public health and related themes. Media Watch is not intended to be exhaustive, but indicative of themes and issues CVEP is actively tracking. This section will grow from an initial base of newspapers, magazines and blog sources, and is segregated from Journal Watch above which scans the peer-reviewed journal ecology.
We acknowledge the Western/Northern bias in this initial selection of titles and invite suggestions for expanded coverage. We are conservative in our outlook in adding news sources which largely report on primary content we are already covering above. Many electronic media sources have tiered, fee-based subscription models for access. We will provide full-text where content is published without restriction, but most publications require registration and some subscription level.
The Atlantic
http://www.theatlantic.com/magazine/
Accessed 20 Feb 2021
Health
A Quite Possibly Wonderful Summer
Families will gather. Restaurants will reopen. People will travel. The pandemic may feel like it’s behind us—even if it’s not.
James Hamblin
February 19, 2021
BBC
http://www.bbc.co.uk/
Accessed 20 Feb 2021
[No new, unique, relevant content]
The Economist
http://www.economist.com/
Accessed 20 Feb 2021
Feb 20th 2021
Sinovacillate
China’s roll-out of covid-19 vaccines is slower than planned
Production bottlenecks and a perceived lack of urgency don’t help
Financial Times
https://www.ft.com/
Accessed 20 Feb 2021
News in-depth Covid-19 vaccines
Racial inequality plagues US vaccine rollout
February 20, 2021
Argentina
Argentina’s health minister fired in ‘VIP vaccines’ scandal
…Argentina’s president fired the country’s health minister on Friday after he was revealed to have personally helped to arrange Covid-19 vaccines for VIPs with government connections. Some of the jabs…
February 20, 2021
Coronavirus Business Update
G7 pledges faster vaccine rollout to developing world
February 19, 2021
Tanzania
Tanzania looks to divine providence as government shuns Covid vaccines
February 19, 2021
Covid-19 vaccines
US will not send vaccines to developing countries until supply improves
…The US will not donate any coronavirus vaccine doses to developing countries until there is a plentiful supply of jabs in the US, Biden administration officials said on Thursday in a firm rejection of…
February 18, 2021
Top of Form
Bottom of Form
Coronavirus pandemic
Taiwan accuses China of blocking efforts to buy Covid vaccines
…Taiwan has accused China of obstructing its efforts to buy coronavirus vaccines from BioNTech, in the latest example of a political dispute that threatens the fight against the pandemic. Taipei was on…
February 18, 2021
Top of Form
Bottom of Form
Top of Form
Bottom of Form
Forbes
http://www.forbes.com/
Accessed 20 Feb 2021
Feb 19, 2021
Pfizer CEO Cautions Against Delaying Second Covid-19 Vaccine Shot
Pfizer CEO Albert Bourla said Friday there wasn’t clear evidence yet on whether it would be safe to forgo a second dose of its coronavirus vaccine or delay administering it, a day after a study showed robust immunity after just one shot.
B y Gina Heeb Forbes Staff
Feb 19, 2021
Variants Could Cause A Rapid Rise In Covid-19 Cases In The U.S. Unless We Implement These Public Health Measures
Two new CDC reports have been released warning that the new variants could lead to a rapid rise in Covid-19 cases in the U.S. In this piece, I outline the public health measures to prevent this.
By William A. Haseltine Contributor
Foreign Affairs
http://www.foreignaffairs.com/
Accessed 20 Feb 2021
[No new, unique, relevant content]
Foreign Policy
http://foreignpolicy.com/
Accessed 20 Feb 2021
Latin America
Mexico Slams Vaccine ‘Hoarding’
Pledges of global vaccine solidarity have been slow to produce results.
By Catherine Osborn
| February 19, 2021, 8:00 AM
G-7 Scrambles for Global Vaccine Plan
After months of warnings, the group of wealthy nations have begun to put forward solutions to the lopsided distribution of coronavirus vaccines.
Morning Brief | Colm Quinn
Countries Are Ramping Up Vaccinations. What About Refugees?
With millions stranded in camps with limited access to shots, there’s a big problem looming in the world’s quest to quash COVID-19.
Report | Christina Lu
The Guardian
http://www.guardiannews.com/
Accessed 20 Feb 2021
[No new, unique, relevant content]
New Yorker
http://www.newyorker.com/
Accessed 20 Feb 2021
The New Yorker Interview
Atul Gawande on COVID-Vaccine Distribution and When Normalcy Might Return
The New Yorker staff writer assesses the vaccination campaign so far, new mutations, and when it might be possible to enter public spaces safely without a mask.
By David Remnick February 19, 2021
New York Times
http://www.nytimes.com/
Accessed 20 Feb 2021
Middle East
Israel Secretly Agrees to Fund Vaccines for Syria as Part of Prisoner Swap
To secure the release of an Israeli civilian held in Syria, Israel agreed to finance a supply of Russian-made Covid-19 vaccines for Damascus, an official said.
By Patrick Kingsley, Ronen Bergman and Andrew E. Kramer
PRINT EDITION February 21, 2021
Canada
Why Canada’s Vaccine Rollout Slowed Down
The government did not promise mass inoculations during the first few months, but reductions and suspensions of shipments have created vaccine anxiety.
By Ian Austen
Europe
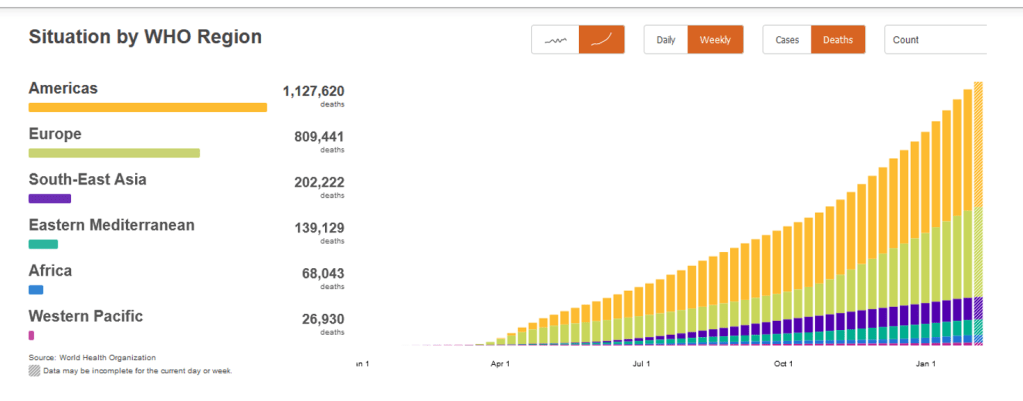
W.H.O. Warns of Unequal Vaccine Distribution
The World Health Organization on Friday warned that the unequal distribution of vaccines across the globe could further the spread of the coronavirus.
By Reuters
Europe
Russia Is Offering to Export Hundreds of Millions of Vaccine Doses, but Can It Deliver?
The Kremlin has scored propaganda points and bolstered several longstanding foreign policy goals by offering its Sputnik V vaccine around the world. But it has limited production capacity.
By Andrew E. Kramer
World
The Vatican is promoting vaccinations but won’t punish those who decline them.
The Vatican said it had issued rules to protect its employees after criticism arose over a decree suggesting that those who didn’t get vaccinated could lose their jobs.
By Elisabetta Povoledo
Middle East
As Israel Reopens, ‘Whoever Does Not Get Vaccinated Will Be Left Behind’
New government and business initiatives are moving in the direction of a two-tier system for the vaccinated and unvaccinated, raising legal, moral and ethical questions.
By Isabel Kershner
Washington Post
https://www.washingtonpost.com/
Accessed 20 Feb 2021
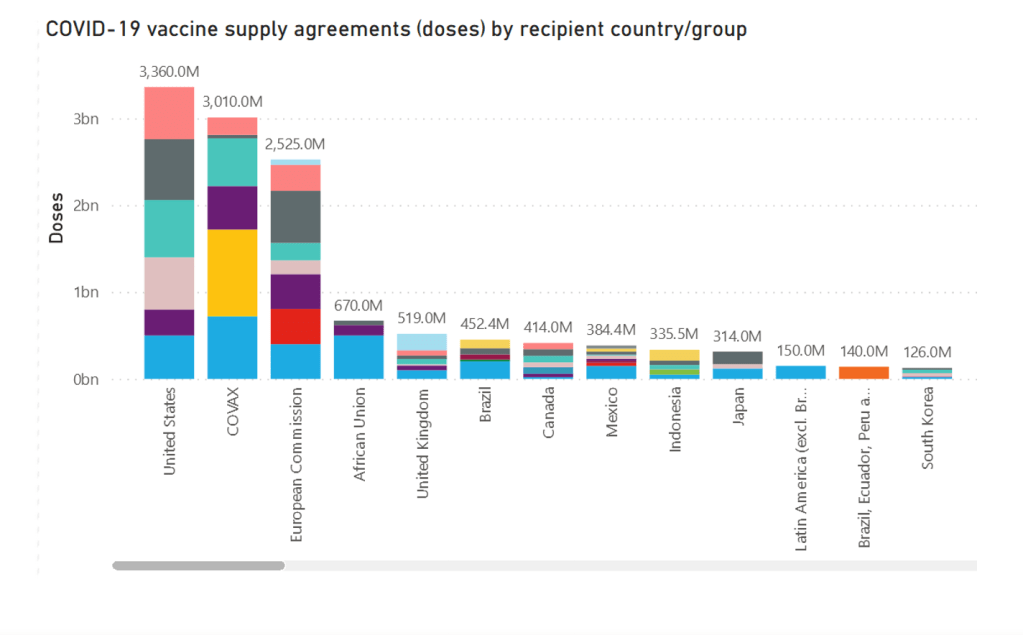
Global vaccine inequality runs deep. Some countries say intellectual property rights are part
Miriam Berger · World · Feb 20, 2021
Israel agrees to vaccinate Palestinian workers, Palestinian officials say
…120,000 doses of the Moderna vaccine in January. It was unclear what vaccines would go to the Palestinians…
Steve Hendrix · Feb 20, 2021
WHO says more than 11,000 Ebola vaccines will go to Guinea
… she said. The vaccination campaign could start as early as Monday. “Thirty vaccination experts have already…
· Feb 18, 2021