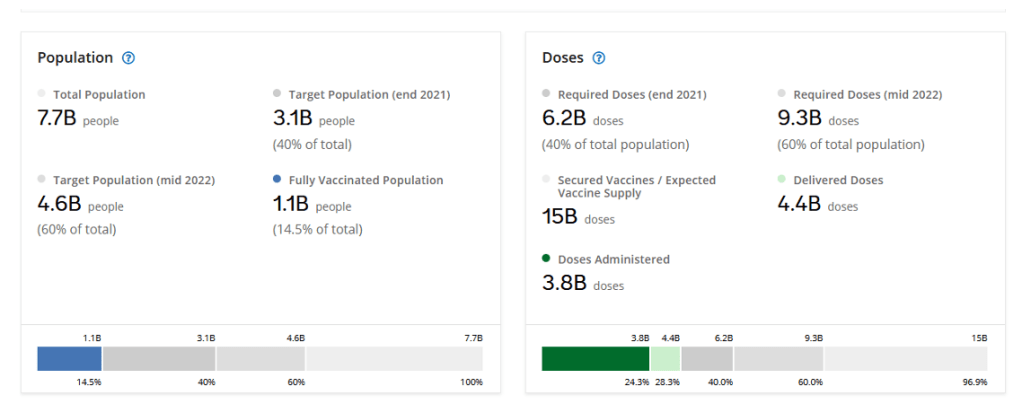
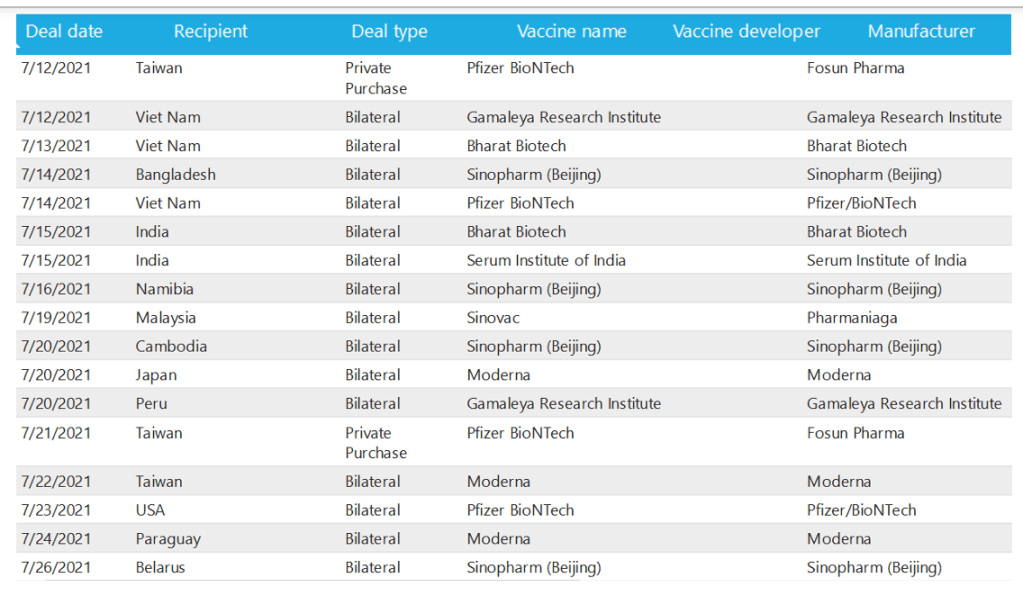
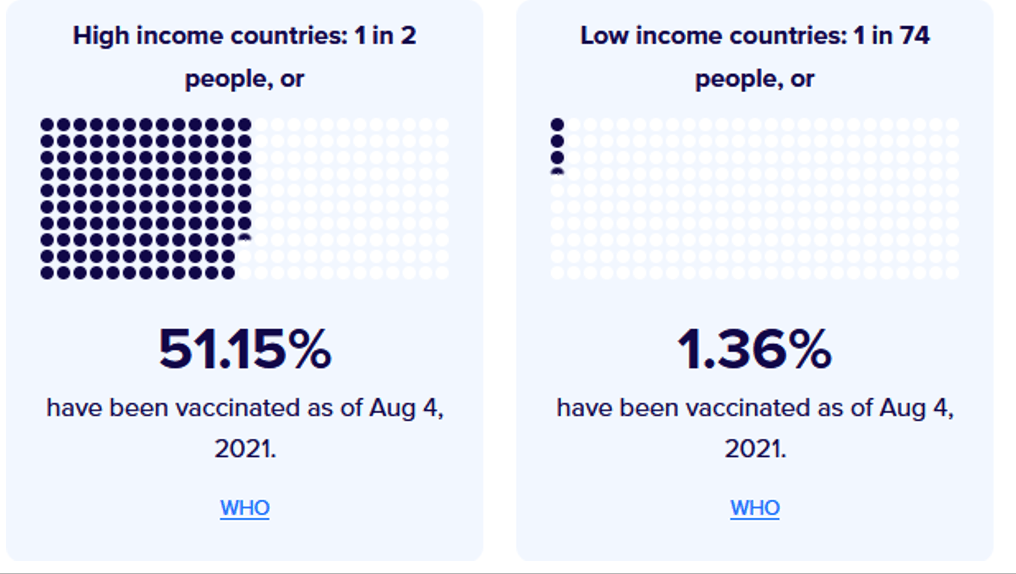
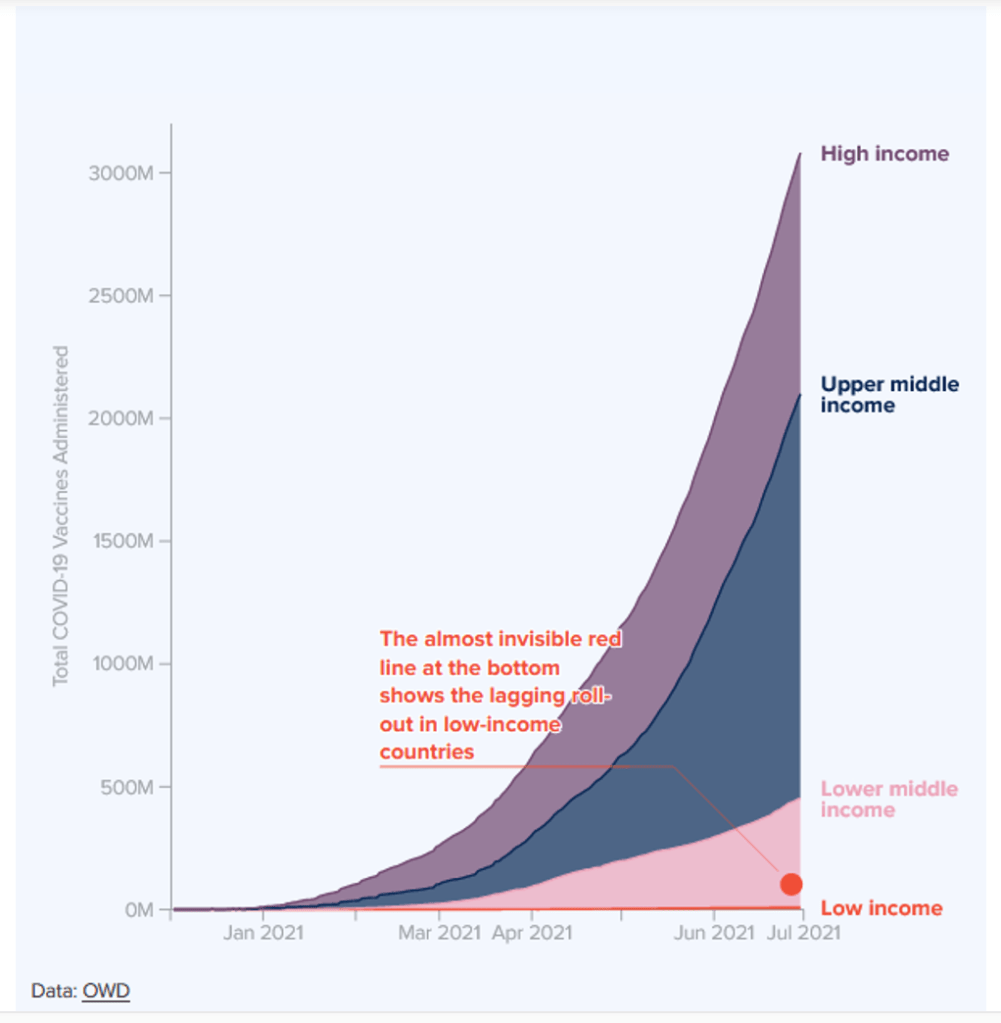
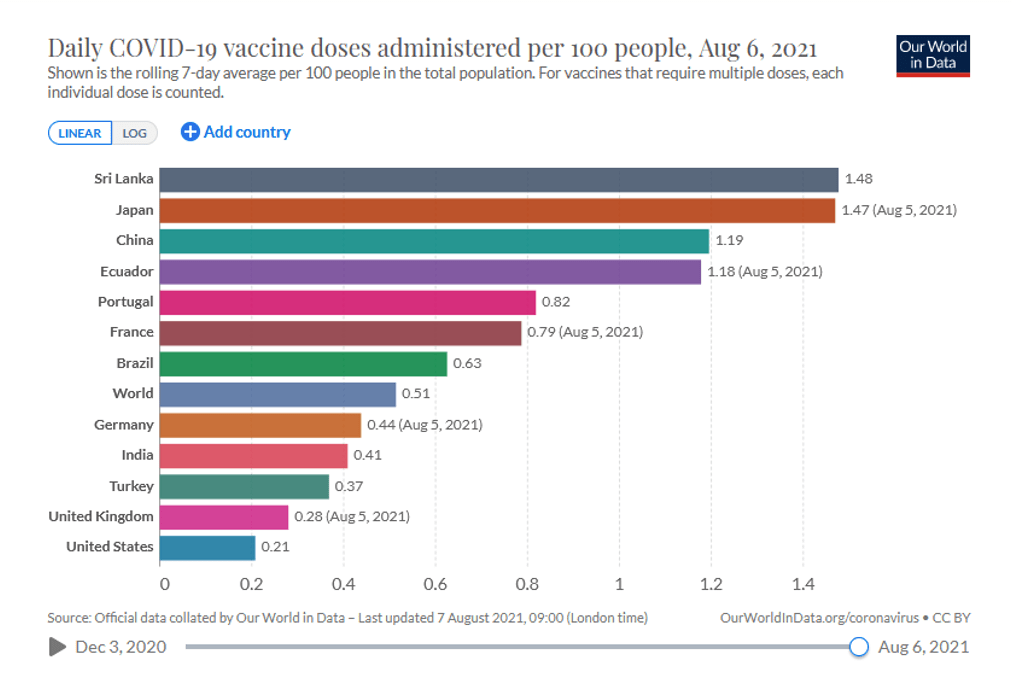
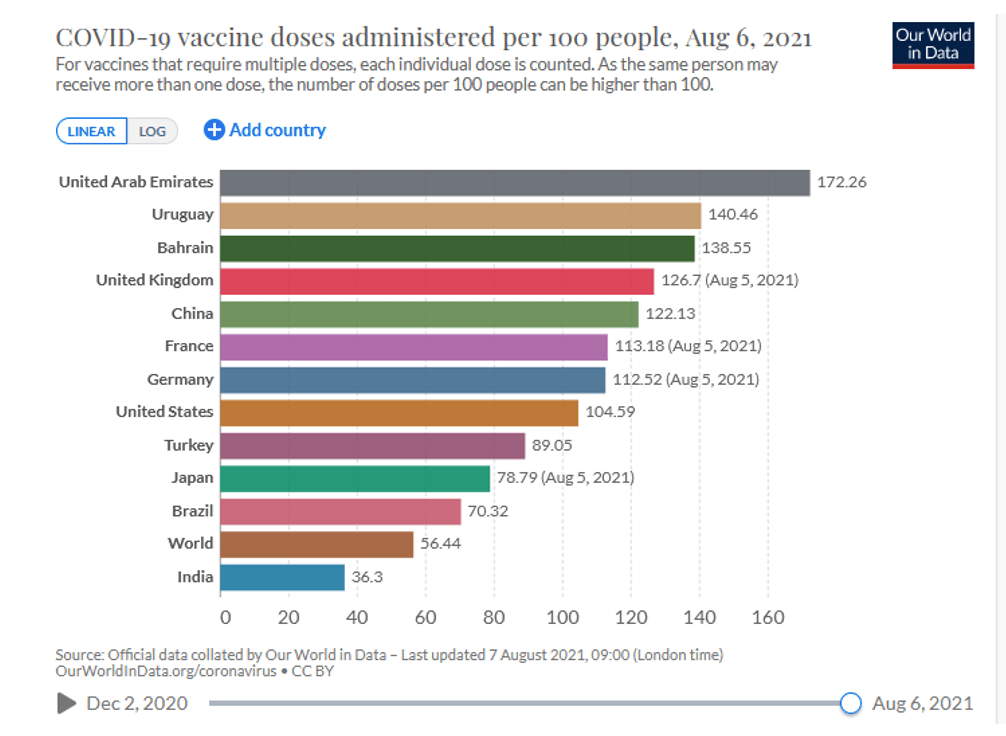
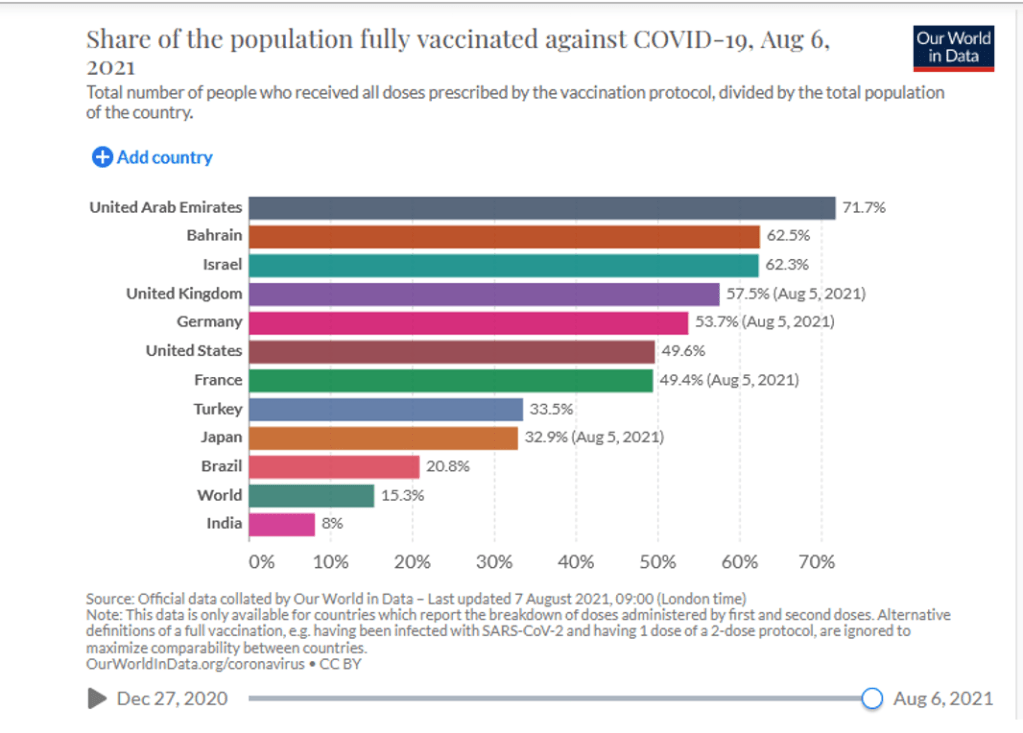
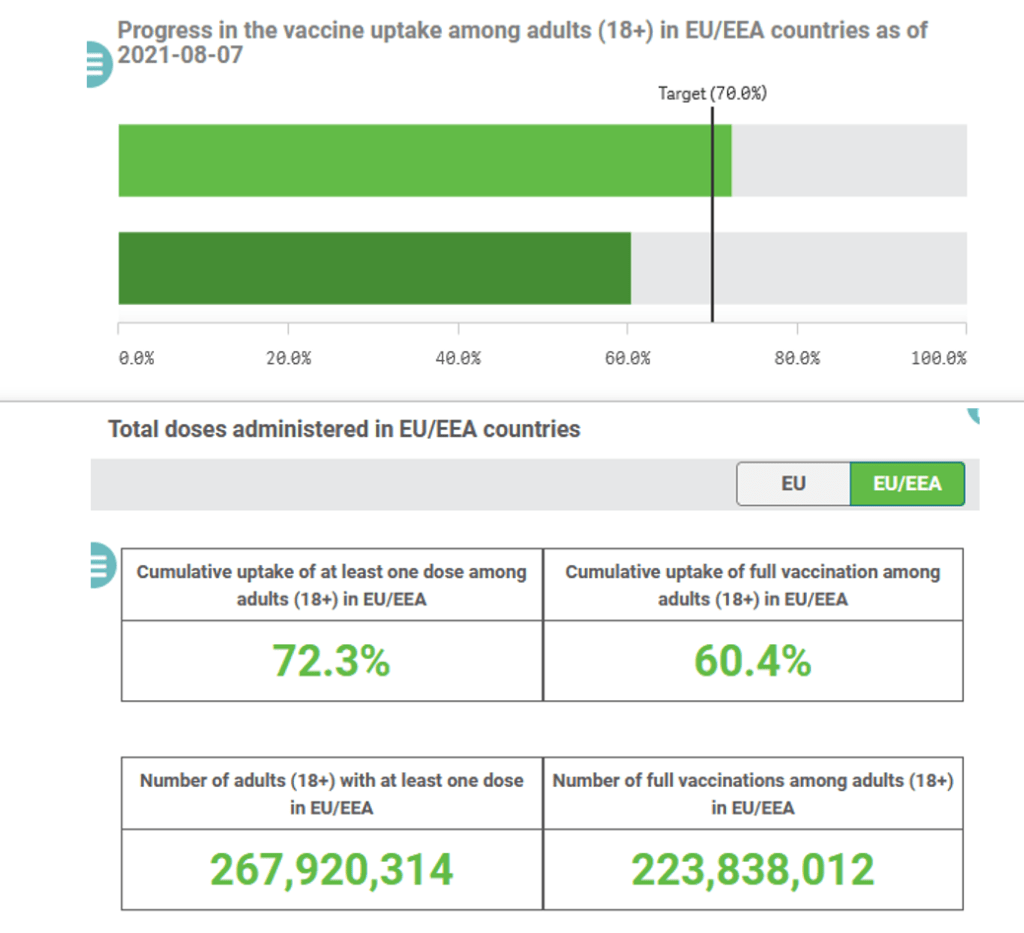
Organization Announcements
Editor’s Note:
Careful readers will note that the number and range of organizations now monitored in our Announcements section below has grown as the impacts of the pandemic have spread across global economies, supply chains and programmatic activity of multilateral agencies and INGOs.
Paul G. Allen Frontiers Group [to 7 Aug 2021]
https://alleninstitute.org/what-we-do/frontiers-group/news-press/
News
No new digest content identified.
BARDA – U.S. Department of HHS [to 7 Aug 2021]
https://www.phe.gov/about/barda/Pages/default.aspx
News
No new digest content identified.
BMGF – Gates Foundation [to 7 Aug 2021]
https://www.gatesfoundation.org/ideas/media-center
Press Releases and Statements
No new digest content identified.
Bill & Melinda Gates Medical Research Institute [to 7 Aug 2021]
https://www.gatesmri.org/
The Bill & Melinda Gates Medical Research Institute is a non-profit biotech organization. Our mission is to develop products to fight malaria, tuberculosis, and diarrheal diseases—three major causes of mortality, poverty, and inequality in developing countries. The world has unprecedented scientific tools at its disposal; now is the time to use them to save the lives of the world’s poorest people
No new digest content identified.
CARB-X [to 7 Aug 2021]
https://carb-x.org/
News
07.29.2021 |
CARB-X celebrates five years of progress in early-stage product development against antibiotic-resistant bacteria
CARB-X, a global non-profit partnership led by Boston University, is celebrating five years of progress in funding and supporting the development of innovative products targeting antibiotic-resistant bacteria. Since it was founded in July 2016, CARB-X has invested $361 million in non-dilutive funding to develop innovative therapeutics including new classes of antibiotics and non-traditional agents, vaccines and other preventatives such as CRISPR-phage, microbiome-modifying agents and antibodies, and rapid diagnostics.
Center for Vaccine Ethics and Policy – GE2P2 Global Foundation [to 7 Aug 2021]
https://centerforvaccineethicsandpolicy.net/
News/Analysis/Statements
:: Past weekly editions and posting of all segments of Vaccines and Global Health: The Week in Review are available here.
:: [NEW] Informed Consent: A Monthly Review – August 2021 is now posted here
CEPI – Coalition for Epidemic Preparedness Innovations [to 7 Aug 2021]
http://cepi.net/
Latest News
Efforts to advance Rift Valley fever vaccines progress with new CEPI partnership
Serum material donated from patients recovered from Rift Valley fever will support the development of a reference tool used to assess Rift Valley fever vaccines undergoing clinical trials.
End Pandemics
06 Aug 2021
CEPI and Universiti Malaya announce new collaboration to advance Nipah virus research
The research aims to characterize and better understand immune responses generated against the virus – one of the deadliest pathogens known to infect humans.
End Pandemics
29 Jul 2021
Largest ever Lassa fever study expands to more countries in West Africa
Up to 23,000 participants will be enrolled into the research programme in Benin, Guinea, Liberia, and Sierra Leone, as well as Nigeria, which began collecting data in December 2020.
End Pandemics
28 Jul 2021
CIOMS – COUNCIL FOR INTERNATIONAL ORGANIZATIONS OF MEDICAL SCIENCES [to 7 Aug 2021]
https://cioms.ch/
News; Publications
No new digest content identified.
DARPA – Defense Advanced Research Projects Agency [to 7 Aug 2021
https://www.darpa.mil/news
News
8/6/2021
DARPA Selects Teams to Develop Novel Therapeutics for Combating Multi-Drug Resistant Microbial Infections
DARPA today announced that it has selected three performer teams to support the Harnessing Enzymatic Activity for Lifesaving Remedies (HEALR) program. Groups from Yale University, University of Washington, and Broad Institute plan to utilize a new therapeutic approach and novel protein degradation strategies/modalities to permit a flexible and rapid response for targeting emerging microbial threats.
Duke Global Health Innovation Center [to 7 Aug 2021]
https://dukeghic.org/
Our Blog
No new digest content identified.
EDCTP [to 7 Aug 2021]
http://www.edctp.org/
The European & Developing Countries Clinical Trials Partnership (EDCTP) aims to accelerate the development of new or improved drugs, vaccines, microbicides and diagnostics against HIV/AIDS, tuberculosis and malaria as well as other poverty-related and neglected infectious diseases in sub-Saharan Africa, with a focus on phase II and III clinical trials
No new digest content identified.
Emory Vaccine Center [to 7 Aug 2021]
http://www.vaccines.emory.edu/
Vaccine Center News
COVID-19 survivors may possess wide-ranging resistance to the disease
Woodruff Health Sciences Center | July 22, 2021
European Vaccine Initiative [to 7 Aug 2021]
http://www.euvaccine.eu/
Latest News
EVI to lead Inno4vac, a new European public-private partnership to innovate vaccine development
The Innovative Medicines Initiative 2 (IMI2) Joint Undertaking mobilised more than € 33 million to support Inno4vac, an innovative public-private partnership to accelerate vaccine R&D timelines. It will focus on the design and application of new and highly advanced predictive models to allow a faster development and manufacturing of novel vaccines.
July 29th, 2021
FDA [to 7 Aug 2021]
https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/default.htm
Press Announcements
August 6, 2021 – Coronavirus (COVID-19) Update: August 6, 2021
August 3, 2021 – Coronavirus (COVID-19) Update: August 3, 2021
:: In mid-July, the FDA held a stakeholder call to discuss COVID-19 vaccines, including preliminary reports of Guillain-Barré Syndrome following Janssen (Johnson & Johnson) COVID-19 vaccination. Acting FDA Commissioner Janet Woodcock, M.D. and the Director of FDA’s Center for Biologics Evaluation and Research, Peter Marks, M.D., Ph.D. were featured speakers. The call can be found on the FDA’s YouTube pageExternal Link Disclaimer.
July 30, 2021 – Coronavirus (COVID-19) Update: July 30, 2021
:: Today, the FDA revised the Emergency Use Authorization (EUA) for REGEN-COV (casirivimab and imdevimab, administered together) to add an authorization of REGEN-COV for emergency use as post-exposure prophylaxis (prevention) for COVID-19 in adults and pediatric individuals (12 years of age and older weighing at least 40 kilograms) who are at high risk for progression to severe COVID-19, including hospitalization or death.
:: On Wednesday, the FDA authorized an extension for the shelf life of the refrigerated Janssen (Johnson & Johnson) COVID-19 Vaccine, allowing the product to be stored at 2-8 degrees Celsius for six months. This extension from four and a half months to six months was granted following a thorough review of data submitted by Janssen and applies to all refrigerated vials of Janssen COVID-19 Vaccine that have been held in accordance with the manufacturer’s storage conditions. The concurrence letter can be read here.
Fondation Merieux [to 7 Aug 2021]
http://www.fondation-merieux.org/
News, Events
No new digest content identified.
Gavi [to 7 Aug 2021]
https://www.gavi.org/
News Releases
6 August 2021
China pledges US$ 100 million towards equitable access to COVID-19 vaccines for lower-income countries
[See COVID above for detail]
5 August 2021
First doses donated by Spain through COVAX arrive to Peru, Guatemala, Paraguay and Nicaragua
4 August 2021
New financing agreement boost for malaria vaccine
30 July 2021
First Swedish doses delivered through COVAX
30 July 2021
First deliveries of Norway-donated COVID-19 vaccines to COVAX
26 July 2021
COVAX and World Bank to Accelerate Vaccine Access for Developing Countries
GHIT Fund [to 7 Aug 2021]
https://www.ghitfund.org/newsroom/press
GHIT was set up in 212 with the aim of developing new tools to tackle infectious diseases that
No new digest content identified.
Global Fund [to 7 Aug 2021]
https://www.theglobalfund.org/en/news/
News & Stories
No new digest content identified.
Global Research Collaboration for Infectious Disease Preparedness [GloPID-R] [to 7 Aug 2021]
https://www.glopid-r.org/news/
News
No new digest content identified.
Hilleman Laboratories [to 7 Aug 2021]
http://www.hillemanlabs.org/
Website reports “under maintenance” at inquiry
Human Vaccines Project [to 7 Aug 2021]
http://www.humanvaccinesproject.org/
News
News webpage not responding at inquiry
IAVI [to 7 Aug 2021]
https://www.iavi.org/newsroom
Latest News
FEATURES
August 3, 2021
Europe-Africa research partnership launches HIV vaccine clinical trial in three African countries
Vaccine candidate targets vulnerabilities common across many HIV-1 variants.
The first volunteers have been vaccinated in Lusaka, Zambia, in a Phase I HIV vaccine trial led by a consortium of researchers in Africa and Europe. The trial, known as HIV-CORE 006, will evaluate the safety, tolerability, and immunogenicity of an HIV vaccine candidate known as HIVconsvX. This novel vaccine construct comprises three immunogens that target vulnerable regions common across most HIV variants. Studies using an earlier version of HIVconsvX showed that the most vulnerable parts of HIV neglected by the immune system during natural infection can induce robust immune responses1.
HIV-CORE 006 builds on extensive research expertise, infrastructure, and community engagement programs within IAVI’s network of Africa clinical research center (CRC) partners. The following CRCs are GREAT consortium members and will participate in HIV-CORE 006:
Uganda: MRC/UVRI and LSHTM Uganda Research Unit
Kenya: KAVI-Institute of Clinical Research; KEMRI-Wellcome Trust Research Programme
Zambia: Center for Family Health Research Zambia
The trial is sponsored by the Globally Relevant AIDS Vaccine Europe-Africa Trials Partnership (GREAT)2 consortium and is supported by the European and Developing Countries Clinical Trials Partnership (EDCTP), with co-funding from IAVI and Oxford University…
International Coalition of Medicines Regulatory Authorities [ICMRA]
http://www.icmra.info/drupal/en/news
Selected Statements, Press Releases, Research
Supply Chain Integrity
Recommendations on common technical denominators for track and trace systems to allow for interoperability
August 6, 2021
The International Coalition of Medicines Regulatory Authorities (ICMRA) published recommendations to facilitate the use of track and trace systems at global level. The paper identifies common technical denominators that allow different systems to exchange and use the available information on medicines and their supply chains in order to protect public health.
In this paper, international regulators emphasise that the interoperability of track and trace systems helps to protect public health by improving information sharing in case of quality defects, reducing shortages, contributing to the fight against falsified medicines and supporting pharmacovigilance activities. A common understanding of these potential benefits of interoperability is fundamental to promoting global planning and implementation of interoperable systems for medicines.
The ICMRA paper was open for public consultation from November 2020 to February 2021. The extensive and helpful feedback was carefully analysed and reviewed in order to refine and finalise the recommendations on common technical denominators for track and trace systems. More details on the comments received and the ICMRA analysis of these comments is available in a separate document.
Recommendations on common technical denominators for track and trace systems to allow for interoperability
Overview of responses to public consultation on track and trace recommendations
ICRC [to 7 Aug 2021]
https://www.icrc.org/en/whats-new
Selected News Releases, Statements, Reports
Health Situation in Yemen
Most of the population lack access to health care due to the destruction of the health facilities in their areas or due to lack of financial resources.
05-08-2021 | Article
International Generic and Biosimilar Medicines Association [IGBA]
https://www.igbamedicines.org/
News
No new digest content identified.
IFFIm
http://www.iffim.org/
Press Releases/Announcements
No new digest content identified.
IFRC [to 7 Aug 2021]
http://media.ifrc.org/ifrc/news/press-releases/
Selected Press Releases, Announcements
Armenia, Azerbaijan, Europe, Georgia, Kazakhstan, Montenegro, Russia, Turkey, Ukraine
IFRC: Delta variant a huge threat in Eastern Europe, South Caucasus and Central Asia
Budapest/Geneva, 6 August 2021 – The International Federation of Red Cross and Red Crescent Societies (IFRC) is calling for more assistance and for vaccinations to be stepped up in Eastern Europe, South Caucasus and Central Asia, where rising COVID-19 …
6 August 2021
Algeria, Libya, Middle East and North Africa, Morocco, Tunisia
IFRC: Inclusive vaccination and protection measures urgently needed to stop the new pandemic waves in North Africa
Beirut, 02 August 2021 – The International Federation of Red Cross and Red Crescent Societies (IFRC) in the Middle East and North Africa, is concerned that the increasing COVID-19 transmissions in the region could spark a domino effect with catastrophi …
2 August 2021
Institut Pasteur [to 7 Aug 2021]
https://www.pasteur.fr/en/press-area
Press Documents
No new digest content identified.
IOM / International Organization for Migration [to 7 Aug 2021]
http://www.iom.int/press-room/press-releases
News
No new digest content identified.
IVAC [to 7 Aug 2021]
https://www.jhsph.edu/research/centers-and-institutes/ivac/index.html
Updates; Events
No new digest content identified.
IVI [to 7 Aug 2021]
http://www.ivi.int/
Selected IVI News, Announcements, Events
THECA consortium begins Vi-TT typhoid conjugate vaccine phase IV effectiveness study in Ghana
July 26, 2021
JEE Alliance [to 7 Aug 2021]
https://www.jeealliance.org/
Selected News and Events
No new digest content identified.
Johns Hopkins Center for Health Security [to 7 Aug 2021]
https://www.centerforhealthsecurity.org/news/center-news/
Center News
New Addendum 2021 Report: COVID-19 Planning Guide and Self-Assessment Toolkit for Higher Education
July 30, 2021
MSF/Médecins Sans Frontières [to 7 Aug 2021]
http://www.msf.org/
Latest [Selected Announcements]
Central American Migration
COVID-19 forces thousands of migrants to cross perilous jungle from Colombia to Panama in search of saf…
Project Update 5 Aug 2021
Public health partnership launched to tackle silent epidemic of hepatitis C
Press Release 27 Jul 2021
National Academy of Medicine – USA [to 7 Aug 2021]
https://nam.edu/programs/
Selected News/Programs
Expert Papers from the NAM Identify Lessons Learned and Compelling Needs for Quality, Safety, and Standards Organizations and Biomedical Research after COVID-19
July 26, 2021
In response to the devastating impact of COVID-19 on the American health system, the National Academy of Medicine (NAM) has convened experts in 9 sectors of health, health care, and biomedical research to review how each sector responded to COVID-19, identify challenges encountered in combating the pandemic, and outline what opportunities exist to reinforce, revitalize, and transform the health system. These insights are being released as 9 NAM Perspectives discussion papers between April and August of 2021, and then bundled into a NAM Special Publication titled Emerging Stronger After COVID-19: Priorities for Health System Transformation, scheduled to be released in Fall 2021…
[See COVID above for example]
National Academy of Sciences – USA [to 7 Aug 2021]
http://www.nasonline.org/news-and-multimedia/
News
No new digest content identified.
National Vaccine Program Office – U.S. HHS [to 7 Aug 2021]
https://www.hhs.gov/vaccines/about/index.html
Upcoming Meetings/Latest Updates
No new digest content identified.
NIH [to 7 Aug 2021]
http://www.nih.gov/news-events/news-releases
News Releases
No new digest content identified.
PATH [to 7 Aug 2021]
https://www.path.org/media-center/
Press Releases
PATH welcomes landmark financing agreement for GSK’s malaria vaccine
Seattle, WA, August 4, 2021 — PATH congratulates Gavi, the Vaccine Alliance, GSK, and MedAccess for joining forces to help ensure an ongoing supply of the first malaria vaccine, GSK’s RTS,S/AS01E. This is another important step toward ensuring access to the vaccine for every child that could benefit from it.
PATH has collaborated in the development of the RTS,S/AS01E malaria vaccine since 2001, first with GSK to advance the vaccine through Phase 2 and Phase 3 trials in Africa, and, since 2017, with GSK and the World Health Organization (WHO) to support pilot implementation of the vaccine in areas of Ghana, Kenya, and Malawi. At the same time, PATH continues efforts to accelerate development of next-generation vaccines and biologics, working with a wide range of academic, business, and governmental organizations.
“It is very encouraging to see leaders in the global health community step up to help ensure access to the RTS,S vaccine,” says Carla Botting, Director of Malaria Market Access and Strategy at PATH. “GSK took a bold step when they agreed to donate millions of doses of vaccine for use in the Malaria Vaccine Implementation Program (MVIP). That donation is helping to make it possible to generate data for a potential WHO recommendation for wider use of the vaccine. Now, Gavi and MedAccess are helping to share the risk so that GSK can continue manufacturing the RTS,S antigen, which will help enable early access to the vaccine and the ability to vaccinate millions more children.”…
Sabin Vaccine Institute [to 7 Aug 2021]
http://www.sabin.org/updates/pressreleases
Statements and Press Releases
No new digest content identified.
UNAIDS [to 7 Aug 2021]
http://www.unaids.org/en
Selected Press Releases/Reports/Statements
UNAIDS report shows that people living with HIV face a double jeopardy, HIV and COVID-19, while key populations and children continue to be left behind in access to HIV services
People living with HIV are at a higher risk of severe COVID-19 illness and death, yet the vast majority are denied access to COVID-19 vaccines. Key populations and their sexual partners account for 65% of new HIV infections but are largely left out of both HIV and COVID-19 responses—800 000 children living with HIV are not on the treatment they need to keep them alive
GENEVA, 14 July 2021—The UNAIDS Global AIDS Update 2021, launched today, highlights evidence that people living with HIV are more vulnerable to COVID-19, but that widening inequalities are preventing them from accessing COVID-19 vaccines and HIV services.
Studies from England and South Africa have found that the risk of dying from COVID-19 among people living with HIV was double that of the general population. In sub-Saharan Africa, which is home to two thirds (67%) of people living with HIV, less than 3% had received at least one dose of a COVID-19 vaccine by July 2021. At the same time, HIV prevention and treatment services are eluding key populations, as well as children and adolescents.
COVID-19 vaccines could save millions of lives in the developing world but are being kept out of reach as rich countries and corporations hold on tightly to the monopoly of production and delivery of supplies for profit. This is having a severe impact around the world as health systems in developing countries become overwhelmed, such as in Uganda, where football stadiums are being turned into makeshift hospitals.
“Rich countries in Europe are preparing to enjoy the summer as their populations have easy access to COVID-19 vaccines, while the global South is in crisis,” said Winnie Byanyima, Executive Director of UNAIDS. “We have failed to learn the lessons of HIV, when millions were denied life-saving medicines and died because of inequalities in access. This is totally unacceptable.”
The new UNAIDS report shows how COVID-19 lockdowns and other restrictions have badly disrupted HIV testing—in many countries this has led to steep drops in HIV diagnoses, referrals to care services and HIV treatment initiations. In KwaZulu-Natal, South Africa, for example, there was a 48% drop in HIV testing after the first national lockdown was imposed in April 2020. There were also fewer new HIV diagnoses and a marked drop in treatment initiation. This occurred as 28 000 HIV community health-care workers were shifted from HIV testing to COVID-19 symptom screening.
The report, Confronting inequalities, shows that in 2020 the 1.5 million new HIV infections were predominantly among key populations and their sexual partners. People who inject drugs, transgender women, sex workers and gay men and other men who have sex with men, and the sexual partners of these key populations, accounted for 65% of HIV infections globally in 2020. Key populations accounted for 93% of new HIV infections outside of sub-Saharan Africa, and 35% within sub-Saharan Africa. However, they remain marginalized and largely out of reach of HIV services in most countries…
UNHCR Office of the United Nations High Commissioner for Refugees [to 7 Aug 2021]
http://www.unhcr.org/en-us/media-centre.htmlS
Selected News Releases, Announcements
UNHCR welcomes U.S. continued efforts to implement measures that protect refugees and asylum-seekers
29 Jul 2021
The 1951 Refugee Convention: 70 years of life-saving protection
28 Jul 2021
UNHCR urges states to protect refugees’ rights, not to instrumentalize their plight
27 Jul 2021
UNICEF [to 7 Aug 2021]
https://www.unicef.org/media/press-releases
Press Releases, News Notes, Statements [Selected]
Press release
08/02/2021
AVAT to partner with UNICEF in scaling up vaccine deliveries to African Union Member States
News note
07/27/2021
Geneva Palais briefing note on the current COVID-19-induced education crisis
This is a summary of what was said by UNICEF Spokesperson James Elder – to whom quoted text may be attributed – at today’s press briefing at the Palais des Nations in Geneva
Press release
07/26/2021
UNICEF signs supply agreement for Sinovac COVID-19 vaccine
Unitaid [to 7 Aug 2021]
https://unitaid.org/
Featured News
04 August 2021
Research begins into new digital tools to support patients and healthcare workers through tuberculosis treatment
27 July 2021
Unitaid paving the way for hepatitis C elimination
26 July 2021
Innovative agreement launches affordable, optimal second-line HIV treatment in low- and middle-income countries
Vaccine Equity Initiative [to 7 Aug 2021]
https://vaccineequitycooperative.org/news/
News
No new digest content identified.
Vaccination Acceptance & Demand Initiative [Sabin) [to 7 Aug 2021]
https://www.vaccineacceptance.org/
Announcements
No new digest content identified.
Vaccine Confidence Project [to 7 Aug 2021]
http://www.vaccineconfidence.org/
News, Research and Reports
Coronavirus global impact
Launched April 2, 2020 and recurring every 3 days, Premise Data is utilizing its global network of Contributors to assess economic, social, and health sentiment surrounding the coronavirus (COVID-19).
Vaccine Education Center – Children’s Hospital of Philadelphia [to 7 Aug 2021]
http://www.chop.edu/centers-programs/vaccine-education-center
News
Vaccine Update for Providers
July 2021
New animation! How mRNA vaccines work
Wellcome Trust [to 7 Aug 2021]
https://wellcome.ac.uk/news
News and reports
Q&A
Charlie Weller
Why do we need a new generation of Covid-19 vaccines?
4 August 2021
The first Covid-19 vaccines have been very effective, but there’s plenty of room for improvement, especially for dealing with new variants. Charlie Weller talks about the potential benefits of new generations of Covid-19 vaccines.
Explainer
How can the world adapt to Covid-19 in the long term?
28 July 2021
As the acute phase of the pandemic passes, Covid-19 will remain with us as an endemic disease – still around, but a manageable threat. What does this mean, and what must we do to stop it from erupting again?
The Wistar Institute [to 7 Aug 2021]
https://www.wistar.org/news/press-releases
Press Releases
No new digest content identified.
WFPHA: World Federation of Public Health Associations [to 7 Aug 2021]
https://www.wfpha.org/
Latest News
No new digest content identified.
World Bank [to 7 Aug 2021]
http://www.worldbank.org/en/news/all
Selected News, Announcements
Africa announces the rollout of 400m vaccine doses to the African Union Member States and the Caribbean
ADDIS ABABA, 5 August, 2021 – His Excellency President Cyril Ramaphosa, President of the Republic of South Africa and African Union (AU) COVID-19 Champion, is pleased to announce the start of monthly…
Date: August 05, 2021 Type: Press Release
[See COVID above for detail]
Gulf Economic Update: COVID-19 Pandemic and the Road to Diversification
In this issue of the Gulf Economic Update, the focus is on fiscal revenues and structural reforms including strategic investments in digitalization and telecommunications. Strategic investment in advanced…
Date: August 04, 2021 Type: Publication
World Bank Support to Bangladesh to Address Challenges Created by Influx of Displaced Rohingya Population (FAQs)
1. What is the World Bank’s position on the repatriation of the displaced Rohingya population? The World Bank is helping Bangladesh address the needs of the displaced Rohingya population until their…
Date: August 03, 2021 Type: Statement
World Bank Group Mobilizes Over $29 billion to Support Latin America and the Caribbean Region Respond to Pandemic
WASHINGTON, August 3, 2021—In response to COVID-19 severely damaging the lives and livelihoods of millions of people in Latin America and the Caribbean countries, the World Bank Group deployed a record…
Date: August 02, 2021 Type: Press Release
Joint Statement of the Multilateral Leaders Task Force on COVID-19 Vaccines, Therapeutics, and Diagnostics for Developing Countries following its Second Meeting
New Global Database to Enhance Transparency and Improve Delivery of COVID-19 Tools WASHINGTON, July 30, 2021 — The Task Force on COVID-19 Vaccines, Therapeutics and Diagnostics for Developing Countries…
Date: July 30, 2021 Type: Statement
[See COVID above for detail]
COVAX and World Bank to Accelerate Vaccine Access for Developing Countries
New mechanism builds on Gavi COVAX Advance Market Commitment (AMC) cost-sharing arrangement WASHINGTON, July 26, 2021 – COVAX and the World Bank will accelerate COVID-19 vaccine supply for developing…
Date: July 26, 2021 Type: Press Release
World Customs Organization – WCO [to 7 Aug 2021]
http://www.wcoomd.org/
Latest News – Selected Items
02 August 2021
WCO Secretary General addresses G20 Culture Ministers’ Meeting in Rome
World Organisation for Animal Health (OIE) [to 7 Aug 2021]
https://www.oie.int/en/for-the-media/press-releases/2021/
Press Releases, Statements
Press Release
Urgent action needed to curb the spread of African swine fever in the Americas
30 July 2021
News
Global report indicates decreasing trend in antimicrobials intended for use in the animal sector
27 July 2021
WTO – World Trade Organisation [to 7 Aug 2021]
http://www.wto.org/english/news_e/news_e.htm
WTO News and Events
STDF Annual Report highlights efforts to boost SPS capacity despite pandemic challenges
2 August 2021
The latest annual report from the Standards and Trade Development Facility (STDF), launched on 2 August, highlights the continued efforts by developing and least developed countries to strengthening their food safety, animal and plant health capacity despite the challenges posed by the COVID-19 pandemic.
IMF, World Bank, WHO, WTO launch joint vaccine information website
30 July 2021
The heads of the International Monetary Fund, World Bank Group, World Health Organization and World Trade Organization launched a new website on 30 July which will serve as a platform for information on access to COVID-19 vaccines, therapeutics and diagnostics and on the activities of the organizations in tackling the pandemic.
WTO report: Full trade recovery at risk without equitable vaccine roll-out
29 July 2021
WTO economies from mid-October 2020 to mid-May 2021 exercised trade policy restraint and refrained from an acceleration of protectionism that would have further harmed a world economy reeling from the COVID-19 pandemic, according to the Director-General’s mid-year report on trade-related developments presented to members on 29 July. The report calls on WTO members to ensure that markets remain open and predictable, and warns that failing to ensure wider access to COVID-19 vaccines could undermine the global economic and trade recovery.
::::::
ARM [Alliance for Regenerative Medicine] [to 7 Aug 2021]
Press Releases – Alliance for Regenerative Medicine (alliancerm.org)
Selected Press Releases
No new digest content identified.
BIO [to 7 Aug 2021]
https://www.bio.org/press-releases
Press Releases, Letters, Testimony, Comments [Selected]
No new digest content identified.
DCVMN – Developing Country Vaccine Manufacturers Network [to 7 Aug 2021]
http://www.dcvmn.org/
News; Upcoming events
No new digest content identified.
ICBA – International Council of Biotechnology Associations [to 7 Aug 2021]
https://internationalbiotech.org/news/
News
ICBA Statement on WHO Recommendations on Human Genome Editing
Jul. 28 2021
IFPMA [to 7 Aug 2021]
http://www.ifpma.org/resources/news-releases/
Selected Press Releases, Statements, Publications
R&D-based pharmaceutical industry’s innovative partnerships to meet urgent global supply needs
21 July 2021
IFPMA member companies are at the forefront of the global effort to develop a safe and effective COVID-19 vaccine and scale up manufacturing to ensure equitable access to people around the world. In less than a year, several vaccines candidates have been approved or are in advanced Phase III clinical trials with encouraging results. An impressive and unprecedented manufacturing scale-up is also taking place. Most collaborations – if not all – involved some sort of licensing and transfer of technology, which would not be possible in the absence of a robust global IP system.
International Alliance of Patients’ Organizations – IAPO [to 7 Aug 2021]
https://www.iapo.org.uk/news/topic/6
Press and media [Selected]
No new digest content identified.
PhRMA [to 7 Aug 2021]
http://www.phrma.org/
Latest News [Selected]
Ensuring global vaccine equity requires eliminating trade and regulatory barriers
August 5, 2021
Trade barriers hamper the COVID-19 vaccine manufacturing process and have direct consequences for global vaccine production and supply…
For example, tariffs and export restrictions on finished vaccines slow the global response to COVID-19 by limiting the ability of people in countries throughout the world to acquire life-saving vaccines. Tariffs and export restrictions on raw materials needed for the vaccine production also slow the global response to COVID-19 by disrupting vaccine manufacturing processes and undermining efforts to promote equitable global vaccine distribution.
The vaccine manufacturing process relies on a complex global network of suppliers that source the raw materials and equipment needed for vaccine production. One COVID-19 vaccine, for example, requires 280 components, relies on 86 suppliers in 19 different countries and utilizes three different manufacturing plants for production. Other industries also depend on many of these same raw materials and equipment, which means that vaccine manufacturers must compete for these essential supplies. The imposition of a tariff or export restriction on any one of these components impedes global vaccine production and distribution.
In addition to being dependent on a vast network of raw materials and equipment suppliers, the vaccine production process depends heavily on highly skilled technical workers, trained to execute specialized and critical functions across the vaccine manufacturing lifecycle. Currently, vaccines are being produced in approximately 70 countries, all of which are home to collaborative efforts to close the vaccine gap. A highly skilled workforce is required on the ground wherever vaccine production occurs, and governments should prioritize the movement of these necessary workers for COVID-19 vaccine manufacturing.
These and other trade and regulatory barriers hamper the manufacturing process and have direct consequences for global vaccine production and supply. ..
Blog Pos
Setting the record straight: Addressing common misconceptions about the COVID-19 vaccine
August 4, 2021
We all must do our part to keep our communities safe, and getting vaccinated is the best protection against COVID-19.
Blog Post
Increasing global access to vaccines by optimizing production
July 29, 2021
Companies have established an unprecedented number of new manufacturing partnerships, creating a global network to produce vaccines at unprecedented speed.
Blog Post