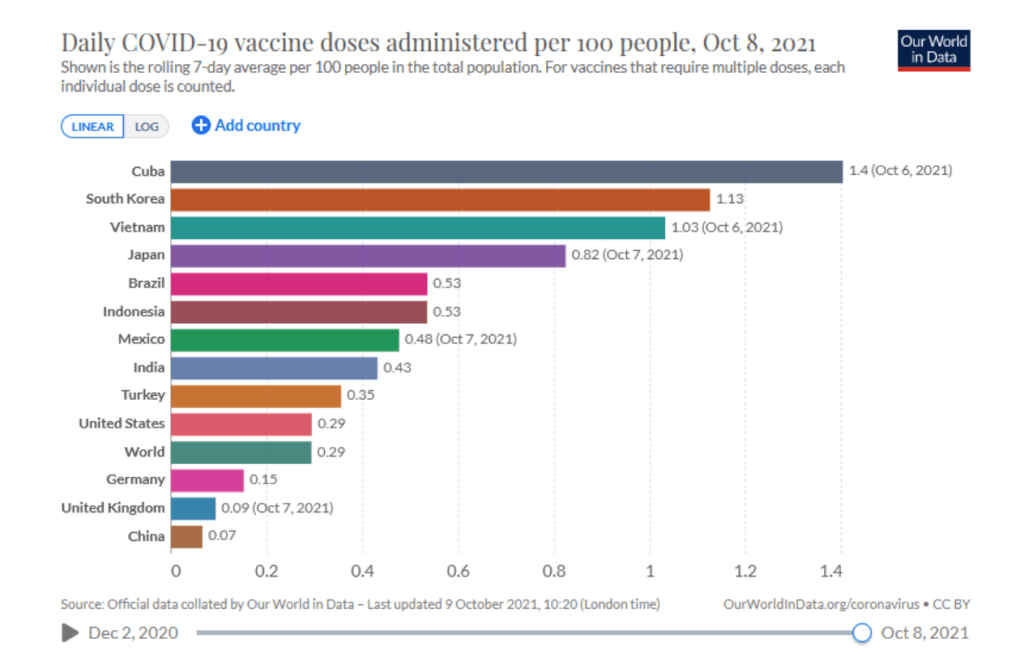
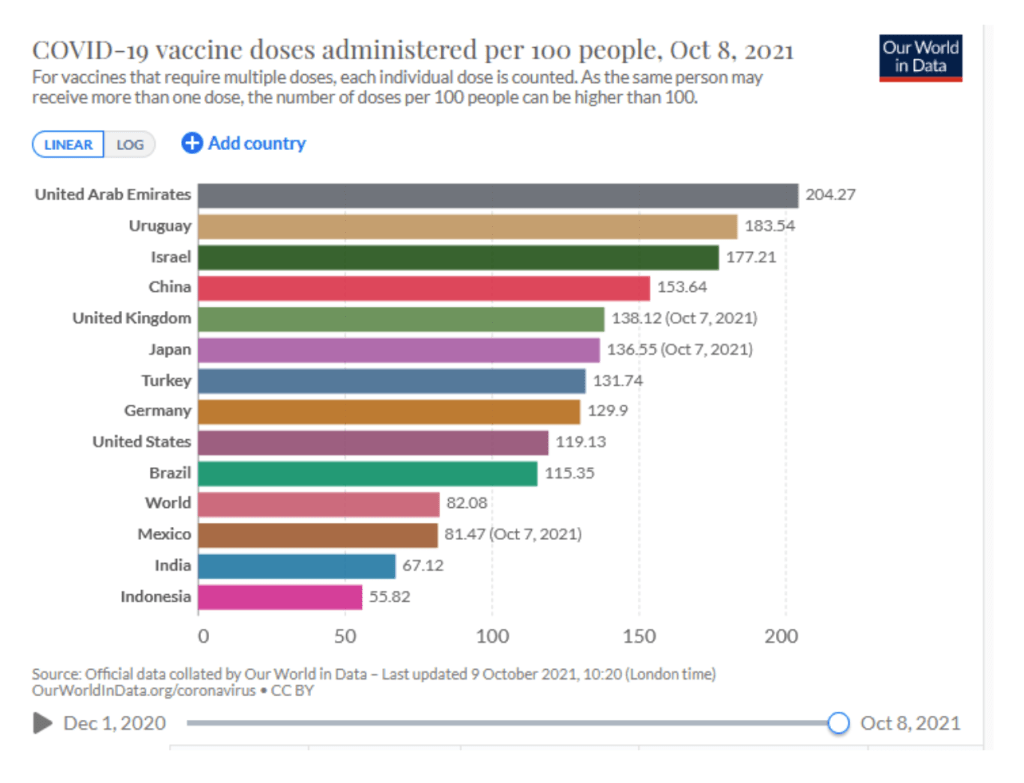
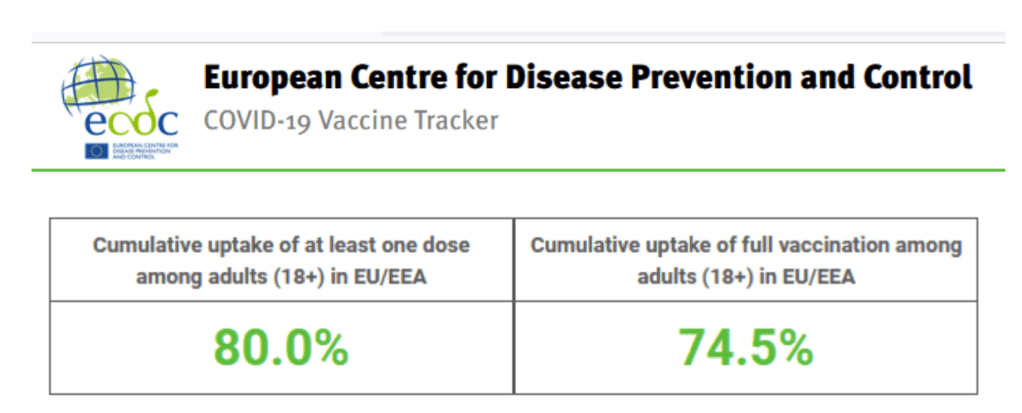
::::::
Organization Announcements
Editor’s Note:
Careful readers will note that the number and range of organizations now monitored in our Announcements section below has grown as the impacts of the pandemic have spread across global economies, supply chains and programmatic activity of multilateral agencies and INGOs.
Paul G. Allen Frontiers Group [to 09 Oct 2021]
https://alleninstitute.org/what-we-do/frontiers-group/new s-press/
News
No new digest content identified.
BARDA – U.S. Department of HHS [to 09 Oct 2021]
https://www.phe.gov/about/barda/Pages/default.aspx
News
October 5, 2021
HHS selects Emory University to demonstrate better approach to disaster medical care
Site becomes fourth to illustrate a regional disaster health response system
To demonstrate a unique approach to improving medical surge and clinical specialty care needed to save lives during a national emergency, the U.S. Department of Health and Human Services’ Office of the Assistant Secretary for Preparedness and Response (ASPR) selected Emory University to lead the latest Regional Disaster Health Response System site…
BMGF – Gates Foundation [to 09 Oct 2021]
https://www.gatesfoundation.org/ideas/media-center
Press Releases and Statements
No new digest content identified.
Bill & Melinda Gates Medical Research Institute [to 09 Oct 2021]
https://www.gatesmri.org/
The Bill & Melinda Gates Medical Research Institute is a non-profit biotech organization. Our mission is to develop products to fight malaria, tuberculosis, and diarrheal diseases—three major causes of mortality, poverty, and inequality in developing countries. The world has unprecedented scientific tools at its disposal; now is the time to use them to save the lives of the world’s poorest people
No new digest content identified.
CARB-X [to 09 Oct 2021]
https://carb-x.org/
News
No new digest content identified.
Center for Vaccine Ethics and Policy – GE2P2 Global Foundation [to 09 Oct 2021]
https://centerforvaccineethicsandpolicy.net/
News/Analysis/Statements
:: Past weekly editions and posting of all segments of Vaccines and Global Health: The Week in Review are available here.
:: [NEW] Informed Consent: A Monthly Review – October 2021 is now posted here
CEPI – Coalition for Epidemic Preparedness Innovations [to 09 Oct 2021]
http://cepi.net/
Latest News
University of Oxford’s Prof. Dame Sarah Gilbert and CEPI’s Dr. Richard Hatchett: “No one is safe, until we are all safe”
In a jointly authored letter, published in Science Translational Medicine, they call for urgent action to address the ongoing disparity in COVID-19 vaccination levels in low-income countries compared to high-income countries.
06 Oct 2021
Further vaccine R&D is critical to end the devastating COVID-19 pandemic
Increased investment in ongoing vaccine R&D efforts will enable COVAX to deliver on its promises and ensure that our vaccines remain safe and effective against the emerging SARS-CoV-2 strains.
Blog
05 Oct 2021
DARPA – Defense Advanced Research Projects Agency [to 09 Oct 2021
https://www.darpa.mil/news
News
No new digest content identified.
Duke Global Health Innovation Center [to 09 Oct 2021]
https://dukeghic.org/
Our Blog
No new digest content identified.
EDCTP [to 09 Oct 2021]
http://www.edctp.org/
The European & Developing Countries Clinical Trials Partnership (EDCTP) aims to accelerate the development of new or improved drugs, vaccines, microbicides and diagnostics against HIV/AIDS, tuberculosis and malaria as well as other poverty-related and neglected infectious diseases in sub-Saharan Africa, with a focus on phase II and III clinical trials
News
07 October 2021
RTS,S/AS01 malaria vaccine: EDCTP contributions and next steps
EDCTP has supported the development of the RTS,S/AS01 malaria vaccine both directly and indirectly through funding clinical research and capacity strengthening in Mozambique, Tanzania, Kenya, Gabon, Ghana and Burkina Faso
Several EDCTP2-funded RTS,S/AS01-related projects are currently ongoing on product-focused implementation research
New and very promising EDCTP-supported early clinical trial results show 77% efficacy for R21/Matrix-M malaria vaccine candidate
Emory Vaccine Center [to 09 Oct 2021]
http://www.vaccines.emory.edu/
Vaccine Center News
No new digest content identified.
European Vaccine Initiative [to 09 Oct 2021]
http://www.euvaccine.eu/
Latest News
No new digest content identified.
Fondation Merieux [to 09 Oct 2021]
http://www.fondation-merieux.org/
News, Events
No new digest content identified.
Gavi [to 09 Oct 2021]
https://www.gavi.org/
News releases
6 October 2021
Gavi, Unitaid and the Global Fund welcome WHO recommendation for world’s first malaria vaccine
[See Milestones above for detail]
6 October 2021
Gulf countries unite to support COVAX
Gulf Cooperation Council (GCC) countries have come together to support global equitable access to COVID-19 vaccines, with pledges now totalling US$ 221 million in funding pledges and US$ 50 million in in-kind support from all six Member States, following Bahrain’s recent commitment of US$ 2.5 million in funding for the Gavi COVAX Advance Market Commitment (Gavi COVAX AMC)
The Kingdom of Bahrain’s Minister of Foreign Affairs, Dr Abdullatif bin Rashid Al Zayani: “The collaborative support of COVAX from GCC Member States is testament to the strength of our relations. It is only through engaging with global health partnerships, such as Gavi, that the international community will be able to suppress COVID-19.”
Dr Seth Berkley, CEO of Gavi, the Vaccine Alliance: “We are delighted to see united support from the GCC Member States for COVAX. This builds on years of growing funding and assistance for Gavi’s routine immunisation programmes.”
The first Gulf nations pledge came from the Kingdom of Saudi Arabia during its G20 Saudi Presidency back in 2020, allocating US$ 150 million to support the Gavi COVAX AMC.
GHIT Fund [to 09 Oct 2021]
https://www.ghitfund.org/newsroom/press
GHIT was set up in 212 with the aim of developing new tools to tackle infectious diseases that
No new digest content identified.
Global Fund [to 09 Oct 2021]
https://www.theglobalfund.org/en/news/
News & Stories
News
Global Fund Crosses US$4 billion Mark in Funding to Support Countries in the Fight Against COVID-19
08 October 2021
News
Global Fund and UNDP Join Efforts to Maintain Access to Essential Health Services in Afghanistan
06 October 2021
On 20 September, the Global Fund and UNDP signed an agreement to provide interim and emergency funding to sustain the delivery of essential health services to the people of Afghanistan. This agreement seeks to provide the resources needed to bridge t…
News
Gavi, Unitaid and the Global Fund Welcome WHO Recommendation for World’s First Malaria Vaccine
06 October 2021
Gavi, the Vaccine Alliance, global health agency Unitaid and the Global Fund to Fight AIDS, Tuberculosis and Malaria welcome the WHO recommendation for wider routine use of the RTS,S malaria vaccine. The recommendation is based on data gathered throu…
[See Milestones above for detail]
Global Research Collaboration for Infectious Disease Preparedness [GloPID-R] [to 09 Oct 2021]
https://www.glopid-r.org/news/
News
No new digest content identified.
Hilleman Laboratories [to 09 Oct 2021]
http://www.hillemanlabs.org/
Website reports “under maintenance” at inquiry
Human Vaccines Project [to 09 Oct 2021]
http://www.humanvaccinesproject.org/
News
No new digest content identified.
IAVI [to 09 Oct 2021]
https://www.iavi.org/newsroom
Latest News
No new digest content identified.
International Coalition of Medicines Regulatory Authorities [ICMRA]
http://www.icmra.info/drupal/en/news
Selected Statements, Press Releases, Research
No new digest content identified.
ICRC [to 09 Oct 2021]
https://www.icrc.org/en/whats-new
Selected News Releases, Statements, Reports
No new digest content identified.
IFFIm
http://www.iffim.org/
Press Releases/Announcements
No new digest content identified.
IFRC [to 09 Oct 2021]
http://media.ifrc.org/ifrc/news/press-releases/
Selected Press Releases, Announcements
08/10/2021
Red Cross set to launch a Humanitarian Logistics Hub in the Southern Cone of the Americas
Buenos Aires, 8 October, 2021 – The Argentine Red Cross (ARC) and the International Federation of Red Cross and Red Crescent Societies (IFRC) will launch a Humanitarian Logistics Hub to expand the Red Cross humanitarian response across Southern Cone countries: Argentina, Bolivia, Brazil, Chile, Paraguay, and Uruguay.
The Humanitarian Hub will have the capacity to pre-position sufficient humanitarian aid to address the needs of up to 10,000 people affected by emergencies and disasters. The Humanitarian Hub is located at Ministro Pistarini International Airport in Buenos Aires…
08/10/2021
Thailand: Nearly 1 million people hit by floods amid COVID surge
Institut Pasteur [to 09 Oct 2021]
https://www.pasteur.fr/en/press-area
Press Documents
No new digest content identified.
IOM / International Organization for Migration [to 09 Oct 2021]
http://www.iom.int/press-room/press-releases
News – Selected
No new digest content identified.
ISC / International Science Council [to 09 Oct 2021]
https://council.science/current/
ISC is a non-governmental organization with a unique global membership that brings together 40 international scientific Unions and Associations and over 140 national and regional scientific organizations including Academies and Research Councils.
News Press
Science as a Global Public Good
The latest position paper published by the International Science Council (ISC) explores the importance of science as a global public good: a source of beneficial and applicable knowledge that is freely available and accessible worldwide, and which can be used by anyone, anywhere, without preventing or impeding its use by others.
PDF: https://council.science/wp-content/uploads/2020/06/Science-as-a-global-public-good_v041021.pdf
IVAC [to 09 Oct 2021]
https://www.jhsph.edu/research/centers-and-institutes/ivac/index.html
Updates; Events
No new digest content identified.
IVI [to 09 Oct 2021]
http://www.ivi.int/
IVI News & Announcements
Remarks by Dr. Jerome Kim, Director General of IVI, during the 2021 IVI State Forum
IVI’s 2021 State Forum highlights opportunities to bridge global vaccine gaps
October 7, 2021, SEOUL, Republic of Korea — The International Vaccine Institute (IVI) hosted its annual State Forum today, bringing together government officials from IVI’s state parties and partner countries to identify gaps in vaccine supply and delivery and to discuss opportunities for building vaccine research, development, and manufacturing capacity in low- and middle-income countries. This year’s dialogue focused on individual country needs and the spectrum of IVI’s capabilities to help solve shared global health challenges…
Johns Hopkins Center for Health Security [to 09 Oct 2021]
https://www.centerforhealthsecurity.org/news/center-news/
Center News
New Report: Masks and Respirators for the 21st Century: Policy Changes Needed to Save Lives and Prevent Societal Disruption
October 5, 2021
The Johns Hopkins Center for Health Security at the Bloomberg School of Public Health released a new report outlining how the federal government can introduce policies that promote a sustainable mask market aimed at improving mask quality and supply and to save lives. The approach outlined in the report touches the development, manufacturing, and stockpiling of masks and respirators for healthcare workers, the nonhealthcare workforce, and the public in the U.S.
The new report, Masks and Respirators for the 21st Century: Policy Changes Needed to Save Lives and Prevent Societal Disruption, points out that the ubiquitous disposable masks and disposable N95 respirators used by healthcare workers have not appreciably improved since the mid-1990s. A confluence of factors currently stymie the mask market, including industrial inertia, lack of competition, complacent consumers, regulatory barriers, an uncertain market, and lack of U.S. government policy…
MSF/Médecins Sans Frontières [to 09 Oct 2021]
http://www.msf.org/
Latest [Selected Announcements]
Democratic Republic of Congo
Healthcare for the community by the community
Project Update 3 Oct 2021
National Academy of Medicine – USA [to 09 Oct 2021]
https://nam.edu/programs/
Selected News/Programs
NAM member David Julius Receives Nobel Prize in Physiology or Medicine
October 4, 2021
The Nobel Assembly at Karolinska Institutet announced today that they have awarded the 2021 Nobel Prize in Physiology or Medicine jointly to David Julius and Ardem Patapoutian for their for their discoveries of receptors for temperature and touch. Julius is a dual member of the National Academy of Medicine and the National Academy of Sciences, […]
National Academy of Sciences – USA [to 09 Oct 2021]
http://www.nasonline.org/news-and-multimedia/
News
No new digest content identified.
National Vaccine Program Office – U.S. HHS [to 09 Oct 2021]
https://www.hhs.gov/vaccines/about/index.html
Upcoming Meetings/Latest Updates
No new digest content identified.
NIH [to 09 Oct 2021]
http://www.nih.gov/news-events/news-releases
News Releases
More than 140,000 U.S. children lost a primary or secondary caregiver due to the COVID-19 pandemic
October 7, 2021 — New study highlights stark disparities in caregiver deaths by race and ethnicity, calls for urgent public health response.
People with substance use disorders may be at higher risk for SARS-CoV-2 breakthrough infections
October 6, 2021 — Co-occurring health disorders appear to contribute to increased risk, NIH study suggests.
NIH supports 106 grants featuring high-risk, high-reward research
October 5, 2021 — The National Institutes of Health awarded 106 grants to support highly innovative and broadly impactful biomedical or behavioral research by exceptionally creative scientists through the Common Fund’s High-Risk, High-Reward Research program. Supported research this year includes understanding how long-term memory might be encoded in the shape of folded DNA in our neurons, mining data from unconventional sources to reveal social determinants of suicide, establishing new paradigms to address the functional consequences of health disparities in drug development, and looking at the impact of high school and collegiate athlete injuries on long-term health. The 106 awards total approximately $329 million over five years, pending availability of funds.
The High-Risk, High-Reward Research program catalyzes scientific discovery by supporting highly innovative research proposals that, due to their inherent risk, may struggle in the traditional peer-review process despite their transformative potential. Program applicants are encouraged to think “outside the box” and pursue trailblazing ideas in any area of research relevant to the NIH’s mission to advance knowledge and enhance health…
Francis Collins to step down as director of the National Institutes of Health
October 5, 2021 — Francis S. Collins, M.D., Ph.D., today announced his decision to end his tenure as the director of the National Institutes of Health by the end of the year. Dr. Collins is the longest serving presidentially appointed NIH director, having served three U.S. presidents over more than 12 years.
“It has been an incredible privilege to lead this great agency for more than a decade,” said Dr. Collins. “I love this agency and its people so deeply that the decision to step down was a difficult one, done in close counsel with my wife, Diane Baker, and my family. I am proud of all we’ve accomplished. I fundamentally believe, however, that no single person should serve in the position too long, and that it’s time to bring in a new scientist to lead the NIH into the future. I’m most grateful and proud of the NIH staff and the scientific community, whose extraordinary commitment to lifesaving research delivers hope to the American people and the world every day.”…
NCI study highlights pandemic’s disproportionate impact on Black, American Indian/Alaska Native, and Latino adults
October 4, 2021 — Investigators analyzed national surveillance data to better understand the impact of the COVID-19 pandemic on excess deaths by racial and ethnic group.
The global COVID-19 pandemic has taken a toll on Black, American Indian/Alaska Native, and Latino individuals in the United States, causing more deaths by population size, both directly and indirectly, in these groups compared with white or Asian individuals. The findings, from a large surveillance study led by researchers at the National Cancer Institute (NCI), part of the National Institutes of Health (NIH), appeared October 5, 2021, in Annals of Internal Medicine.
“Focusing on COVID-19 deaths alone without examining total excess deaths—that is, deaths due to non-COVID-19 causes as well as to COVID-19—may underestimate the true impact of the pandemic,” said Meredith S. Shiels, Ph.D., M.H.S., senior investigator in the Infections and Immunoepidemiology Branch in NCI’s Division of Cancer Epidemiology and Genetics, who led the study. “These data highlight the profound impact of long-standing inequities.”…
PATH [to 09 Oct 2021]
https://www.path.org/media-center/
Press Releases
USAID announces new five-year project to detect unknown viruses with pandemic potential
October 8, 2021 by PATH
SEATTLE, WA – The United States Agency for International Development (USAID) has just launched an ambitious new project that will work with partner countries and the global community to build better preparedness for future global health threats. Discovery & Exploration of Emerging Pathogens – Viral Zoonoses (DEEP VZN), a five-year, approximately $125 million project, will strengthen global capacity to detect and understand the risks of viral spillover from wildlife to humans that could cause another pandemic…
DEEP VZN builds on previous work by significantly scaling up USAID’s efforts to understand where and how viruses spillover from animals to humans. The Washington State University Paul Allen School for Global Health will implement the project with a consortium of partners that includes PATH, the University of Washington, FHI 360, and Washington University in St. Louis. DEEP VZN will partner with researchers and institutions in up to 12 targeted countries in Africa, Asia, and Latin America that have both a high risk for viral spillover and the capacity to safely conduct viral discovery.
The project will focus on finding previously unknown pathogens from three viral families that have a large potential for viral spillover from animals to humans: coronaviruses, the family that includes SARS-CoV-2 the virus that causes COVID-19; filoviruses, such as the Ebola virus; and paramyxoviruses which includes the viruses that cause measles and Nipah…
PATH applauds WHO recommendation for broader use of first malaria vaccine
Vaccine shown to be cost-effective and trusted by caregivers and health care workers
October 6, 2021 by PATH
Seattle, WA – PATH applauds the announcement today that the World Health Organization (WHO) has recommended broader use of the world’s first malaria vaccine, RTS,S/AS01E (RTS,S). This historic recommendation means that the vaccine, which is currently in routine use as part of a pilot program in areas of Ghana, Kenya, and Malawi, can soon be available – as an additional malaria control tool – to more children in these three countries, and in other malaria-endemic nations as well…
The pilot program includes evaluation of the vaccine in routine use. As part of this, PATH has been leading studies on the vaccine’s cost-effectiveness and public health impact and on community acceptance of the vaccine. Findings from these studies, in addition to data on the feasibility of administering four doses of RTS,S, the vaccine’s potential in reducing childhood deaths, and its safety in the context of routine use, informed the WHO recommendation. The pilot program will continue through 2023.
“Using all the information generated by the malaria vaccine pilots, modeling groups have shown that RTS,S would be a cost-effective addition to the suite of currently available malaria interventions,” said Dr. Ashley Birkett, Director of PATH’s Malaria Vaccine Initiative. “Additionally, this work has shown the vaccine could have considerable public health impact, averting approximately one death for every 220 children vaccinated with a minimum of three doses in areas of moderate to high malaria transmission.”…
Sabin Vaccine Institute [to 09 Oct 2021]
http://www.sabin.org/updates/pressreleases
Statements and Press Releases
No new digest content identified.
UNAIDS [to 09 Oct 2021]
http://www.unaids.org/en
Selected Press Releases/Reports/Statements
5 October 2021
Multicountry People Living with HIV Stigma Index 2.0 study launched in Latin America
4 October 2021
Don’t be silent on gender-based violence
4 October 2021
Slow progress on AIDS-related deaths among adolescents
UNHCR Office of the United Nations High Commissioner for Refugees [to 09 Oct 2021]
http://www.unhcr.org/en-us/media-centre.htmlS
Selected News Releases, Announcements
News comment by UN High Commissioner for Refugees Filippo Grandi on solutions for millions of forcibly displaced people from Sudan and South Sudan
5 Oct 2021
Joint Statement by IGAD, UNHCR and the governments of South Sudan and Sudan on the Solutions Initiative for 7 million forcibly displaced people
5 Oct 2021
UNICEF [to 09 Oct 2021]
https://www.unicef.org/media/press-releases
Press Releases, News Notes, Statements [Selected]
Press release
10/05/2021
Half of Afghanistan’s children under five expected to suffer from acute malnutrition as hunger takes root for millions
UNICEF and WFP representatives sound alarm on nutrition crisis for children and mothers following joint visit to Herat
Press release
10/04/2021
Impact of COVID-19 on poor mental health in children and young people ‘tip of the iceberg’
New analysis indicates lost contribution to economies due to mental disorders among young people estimated at nearly $390 billion a year
Unitaid [to 09 Oct 2021]
https://unitaid.org/
Featured News
06 October 2021
GAVI, Unitaid and the Global Fund welcome WHO recommendation for world’s first malaria vaccine
[See Milestones above for detail]
04 October 2021
New paediatric formulation for HIV treatment hits the ground in six African countries
Geneva, 4 October 2021 – On World AIDS Day 2020, Unitaid and the Clinton Health Access Initiative (CHAI) announced a groundbreaking deal that would see the very best HIV treatment made available to the youngest children for the first time.
Access for the youngest children
1.8 million children around the world live with HIV, the majority in low- and middle-income countries. Only 53% of these children are diagnosed and on treatment, while 80,000 babies and toddlers die each year from AIDS.
Ensuring access to treatments specifically designed for children is a key priority for Unitaid – and the agreement announced on December 1st 2020 saw the price for pediatric HIV treatment reduced by 75% with a new 10mg scored, dispersible formulation of dolutegravir.
Unitaid Executive Director Dr Philippe Duneton said: “To see this new paediatric formulation of DTG hitting the ground in six initial countries – and knowing that so many more are to come – is a huge moment for all the partners involved, and the communities that will benefit. Making the very best treatments available to the youngest children is at the heart of what Unitaid does and is vital if we are to achieve the global goals for HIV.”..
04 October 2021
Innovations in paediatric medicines delivery awarded UnitaidExplore funding
Two new awards announced under Unitaid’s agility mechanism, UnitaidExplore; DelSiTech and FluidPharma will each receive investment for innovations to make medicines easier to give to children
Latest call comes in context of Unitaid’s ground-breaking work on paediatric formulations to treat HIV, TB and malaria
Children in low- and middle-income countries have lower treatment coverage and worse health outcomes than adults – a lack of paediatric formulations is a major contributing factor.
Vaccine Equity Cooperative [nee Initiative] [to 09 Oct 2021]
https://vaccineequitycooperative.org/news/
News
No new digest content identified.
Vaccination Acceptance & Demand Initiative [Sabin) [to 09 Oct 2021]
https://www.vaccineacceptance.org/
Announcements
No new digest content identified.
Vaccine Confidence Project [to 09 Oct 2021]
http://www.vaccineconfidence.org/
News, Research and Reports
Coronavirus global impact
Launched April 2, 2020 and recurring every 3 days, Premise Data is utilizing its global network of Contributors to assess economic, social, and health sentiment surrounding the coronavirus (COVID-19).
Vaccine Education Center – Children’s Hospital of Philadelphia [to 09 Oct 2021]
http://www.chop.edu/centers-programs/vaccine-education-center
News
No new digest content identified.
Wellcome Trust [to 09 Oct 2021]
https://wellcome.ac.uk/news
News and reports
Explainer
What treatments are working for Covid-19?
4 October 2021
From existing antivirals to new antibody therapies – researchers are working tirelessly to find the best drugs to treat Covid-19.
The Wistar Institute [to 09 Oct 2021]
https://www.wistar.org/news/press-releases
Press Releases
No new digest content identified.
WFPHA: World Federation of Public Health Associations [to 09 Oct 2021]
https://www.wfpha.org/
Latest News
No new digest content identified.
World Bank [to 09 Oct 2021]
http://www.worldbank.org/en/news/all
Selected News, Announcements
MENA Economic Update: Overconfident: How Economic and Health Fault Lines Left the Middle East and North Africa Ill-Prepared to Face COVID
This edition of the World Bank MENA Economic Update estimates that the Middle East and North Africa (MENA) region’s economies, which contracted by 3.8% in 2020, will grow by 2.8% in 2021. Overall, the…
Date: October 07, 2021 Type: Publication
COVID-19 Stress Tests Region’s Ill-prepared Health Systems: MENA Shows Tenuous, Uneven Recovery in 2021
GDP will grow 2.8% by end 2021 WASHINGTON, October 7, 2021 — Long-term socio-economic trends and underfunded public health systems left the Middle East and North Africa (MENA) region ill-prepared to respond…
Date: October 07, 2021 Type: Press Release
Recovering Growth: Rebuilding Dynamic Post-Covid Economies Amid Fiscal Constraints
Latin America is emerging from the COVID-19 crisis, but the recovery is weaker than expected, and the scars on the economy and society will take years to fade. The need to recover dynamic, inclusive, and…
Date: October 06, 2021 Type: Brief
Urgent reforms needed to boost growth and prevent another Lost Decade in Latin America and the Caribbean
WASHINGTON, October 6, 2021 — The scars from the COVID-19 crisis will take years to fade if countries in Latin America and the Caribbean don’t take immediate steps to boost a lackluster recovery from the…
Date: October 05, 2021 Type: Press Release
New boost to vaccination in Argentina
With almost half of the population having completed their vaccination scheme and the easing of restrictions allowing greater flexibility in social and economic activities, Argentina is beginning to feel…
Date: October 04, 2021 Type: Feature Story
The national vaccination plan recently received a new boost with US$500 million financing from the World Bank, which will allow the purchase of some 40 million vaccines to cover almost 30 percent of the population
World Customs Organization – WCO [to 09 Oct 2021]
http://www.wcoomd.org/
Latest News – Selected Items
05 October 2021
The Americas and Caribbean region discusses on ethics, transparency and integrity
World Organisation for Animal Health (OIE) [to 09 Oct 2021]
https://www.oie.int/en/media/news/
Press Releases, Statements
No new digest content identified.
WTO – World Trade Organisation [to 09 Oct 2021]
http://www.wto.org/english/news_e/news_e.htm
WTO News and Events
WTO issues papers on vaccine inputs tariffs and bottlenecks on critical COVID-19 products
8 October 2021
The WTO Secretariat has published two information notes on issues relating to the manufacturing of COVID-19 vaccines.
[See COVID above for detail]
::::::
ARM [Alliance for Regenerative Medicine] [to 09 Oct 2021]
https://alliancerm.org/press-releases/
Selected Press Releases
No new digest content identified.
BIO [to 09 Oct 2021]
https://www.bio.org/press-releases
Press Releases, Letters, Testimony, Comments [Selected]
No new digest content identified.
DCVMN – Developing Country Vaccine Manufacturers Network [to 09 Oct 2021]
http://www.dcvmn.org/
News; Upcoming events
No new digest content identified.
ICBA – International Council of Biotechnology Associations [to 09 Oct 2021]
https://internationalbiotech.org/news/
News
No new digest content identified.
IFPMA [to 09 Oct 2021]
http://www.ifpma.org/resources/news-releases/
Selected Press Releases, Statements, Publications
No new digest content identified.
International Generic and Biosimilar Medicines Association [IGBA]
https://www.igbamedicines.org/
News
Smart use of off-patent medicines will be critical to rebuild healthcare systems successfully post-Covid (October 2021)
07 October, Athens, Greece.
Health systems have been severely jolted by COVID-19, causing widespread disruption to the provision of health care worldwide. As countries shift to investment in healthcare system recovery and ongoing management of COVID-19, off-patent medicines will be critical to their success.
Off-patent medicines provide gold standard care for the majority of patients to manage chronic conditions worldwide. Generic medicines have been a game changer for cardiac, cancer and bacterial infection control, while biosimilar medicines have improved the lives of those living with inflammatory conditions and certain cancers. The future looks personalised with smart innovation on existing molecules, bringing value added medicines to the centre of patient care.
As health systems develop recovery plans from the pandemic, it is essential that off patent medicines become the focal point of pharmaceutical policies. We have learned valuable lessons from economic crises of the past, including the detrimental impact of cost containment measures. As we look to rebuild from COVID-19, only sustainable policies based on medicines use and uptake, and their value for health systems and patients should under pin future reforms.
Speaking at the Medicines for Europe-IGBA annual conference, Medicines for Europe Interim President Rebecca Guntern commented “The off-patent medicines industry mobilised like never before during COVID-19 and we can be truly proud that we kept supplies of critical medicines going in the face of unprecedented challenges. The focus now should be on making sure that the recovery response is equally strong and thought through, integrating the lessons we all learned in the crisis. Off-patent medicines have always been the foundation of ensuring equitable access, but this is even more so now as we work together to rebuild our healthcare systems. We look forward to being an integral part of that discussion, particularly through the new EU Pharma Strategy and EU Beating Cancer plan”…
…IGBA Chair Sudarshan Jain concluded “The issue of global cooperation in health has never been as needed and prolific as it is now. We must keep this momentum going and ensure that all stakeholders in the healthcare systems of all regions collaborate beyond COVID-19. This is also an important moment in history for regulatory harmonisation and reliance, embracing the digital agenda in healthcare and developing smart health and trade policies world wide.”
International Alliance of Patients’ Organizations – IAPO [to 09 Oct 2021]
https://www.iapo.org.uk/news/topic/6
Press and media [Selected]
No new digest content identified.
PhRMA [to 09 Oct 2021]
http://www.phrma.org/
Latest News [Selected]
No new digest content identified.