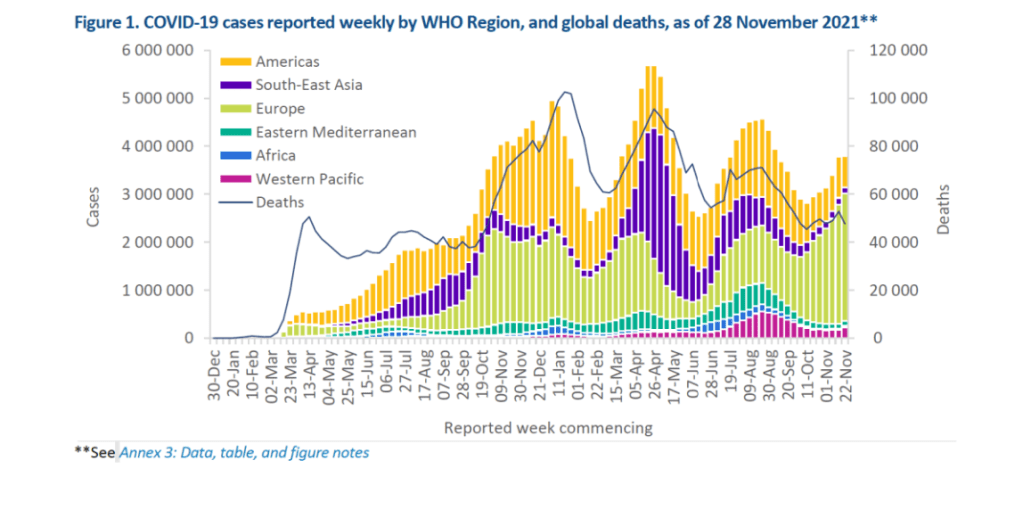
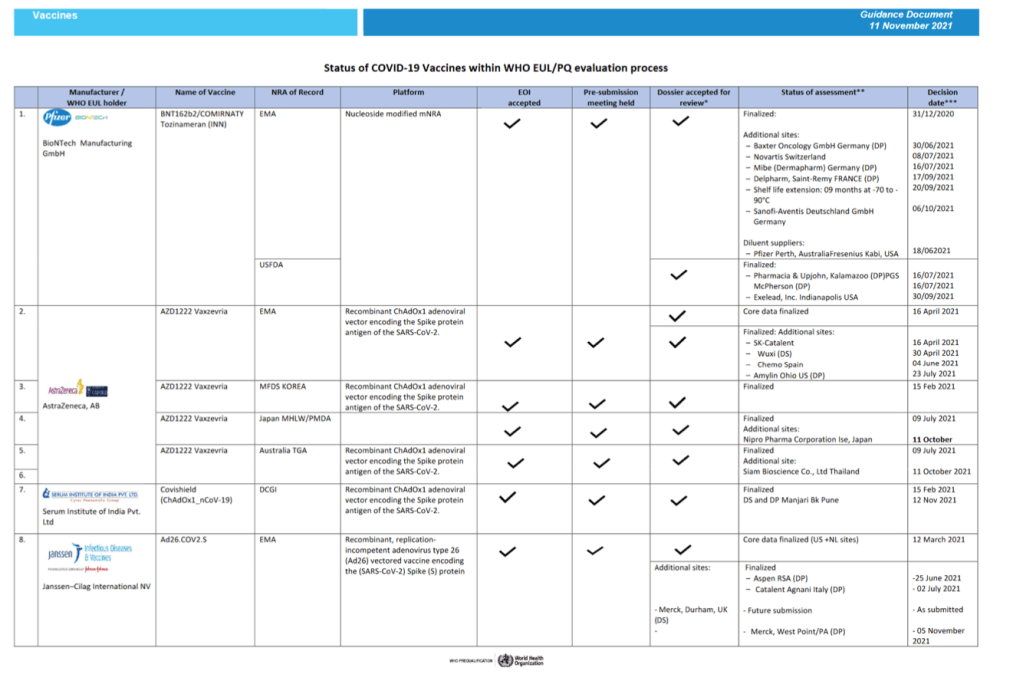
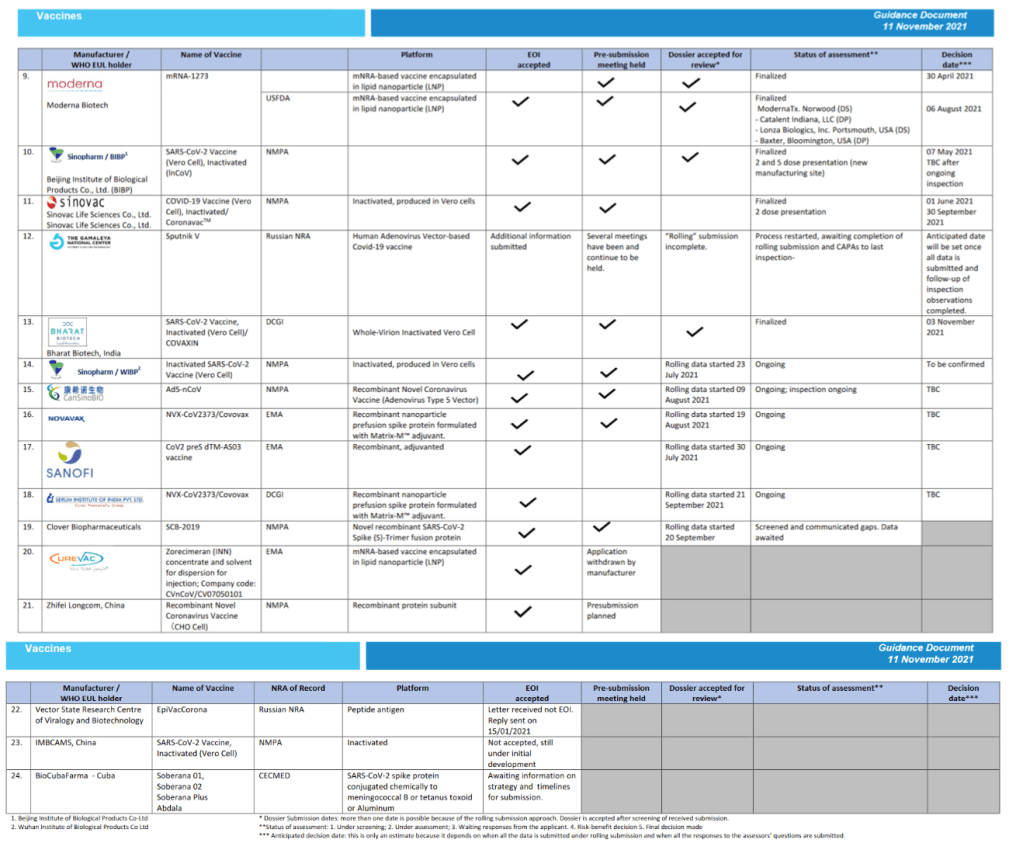
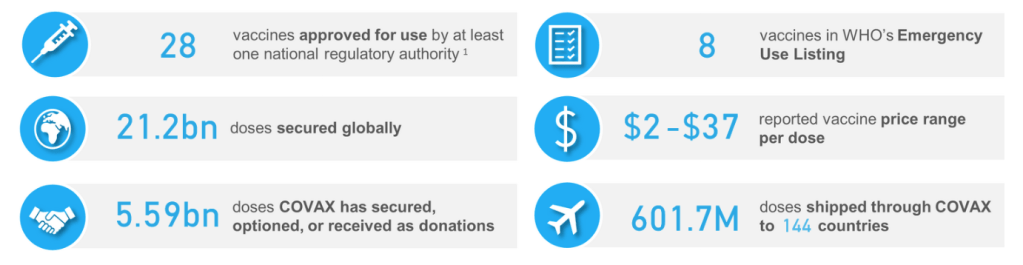
::::::
Organization Announcements
Editor’s Note:
Careful readers will note that the number and range of organizations now monitored in our Announcements section below has grown as the impacts of the pandemic have spread across global economies, supply chains and programmatic activity of multilateral agencies and INGOs.
Paul G. Allen Frontiers Group [to 4 Dec 2021]
https://alleninstitute.org/news-press/
News
$20M awards fund centers’ studies of human brain evolution, animal development
December 2, 2021
Two Allen Discovery Centers enter their next phase of discovery, poised to address large questions about biology
BARDA – U.S. Department of HHS [to 4 Dec 2021]
https://www.phe.gov/about/barda/Pages/default.aspx
News
No new digest content identified.
BMGF – Gates Foundation [to 4 Dec 2021]
https://www.gatesfoundation.org/ideas/media-center
Press Releases and Statements
No new digest content identified.
Bill & Melinda Gates Medical Research Institute [to 4 Dec 2021]
https://www.gatesmri.org/
The Bill & Melinda Gates Medical Research Institute is a non-profit biotech organization. Our mission is to develop products to fight malaria, tuberculosis, and diarrheal diseases—three major causes of mortality, poverty, and inequality in developing countries. The world has unprecedented scientific tools at its disposal; now is the time to use them to save the lives of the world’s poorest people
No new digest content identified.
CARB-X [to 4 Dec 2021]
https://carb-x.org/
News
No new digest content identified.
Center for Vaccine Ethics and Policy – GE2P2 Global Foundation [to 4 Dec 2021]
https://centerforvaccineethicsandpolicy.net/
News/Analysis/Statements
:: Past weekly editions and posting of all segments of Vaccines and Global Health: The Week in Review are available here.
:: Informed Consent: A Monthly Review – December 2021 is now posted here
CEPI – Coalition for Epidemic Preparedness Innovations [to 4 Dec 2021]
http://cepi.net/
Latest News
CEPI and MVC co-fund mix-and-match booster trial of COVID-19 vaccines
Clinical trial will evaluate heterologous and fractional booster doses – or ‘mix-and-match’ booster – combinations of MVC-COV1901 with other COVID-19 vaccines.
02 Dec 2021
Speech made by CEPI CEO, Dr Richard Hatchett, on the emergence of Omicron variant at today’s World Health Assembly special session
Dr Richard Hatchett, CEO of CEPI, addressed today’s special session of the World Health Assembly—on behalf of the Access to COVID-19 Tools Accelerator.
“…South Africa and Botswana’s efficient surveillance, early detection, and warning, and sharing of information have granted the world precious time. Time to implement containment measures and time to prepare. Our industry partners are now intensively investigating whether our current vaccines have been compromised and consulting with regulators and WHO as they develop new ones just in case.
“As you embark on this historic Special Session, I would urge you to keep this example, which is right in front of you, in mind.
“The world is at a crossroads. We know we will face future global infectious disease emergencies and potential pandemic threats. That is a certainty. What happens when these threats materialize, however, is up to us – and in a very tangible sense, up to you.
“We urgently need a global framework or convention on pandemic preparedness and response if we are to have any hope of preventing future pandemics, accelerating the global availability of critical medical countermeasures, and ensuring more just outcomes than we have achieved with COVID…”
DARPA – Defense Advanced Research Projects Agency [to 4 Dec 2021
https://www.darpa.mil/news
News
Our Blog
No new digest content identified.
Duke Global Health Innovation Center [to 4 Dec 2021]
https://dukeghic.org/
Our Blog
No new digest content identified.
EDCTP [to 4 Dec 2021]
http://www.edctp.org/
The European & Developing Countries Clinical Trials Partnership (EDCTP) aims to accelerate the development of new or improved drugs, vaccines, microbicides and diagnostics against HIV/AIDS, tuberculosis and malaria as well as other poverty-related and neglected infectious diseases in sub-Saharan Africa, with a focus on phase II and III clinical trials
News
01 December 2021
World AIDS Day 2021: tackling inequalities
Emory Vaccine Center [to 4 Dec 2021]
http://www.vaccines.emory.edu/
Vaccine Center News
No new digest content identified.
European Vaccine Initiative [to 4 Dec 2021]
http://www.euvaccine.eu/
Latest News News, Events
EVI
World AIDS Day 2021: End inequalities. End AIDS
1 December 2021 What is world AIDS
Fondation Merieux [to 4 Dec 2021]
http://www.fondation-merieux.org/
News, Events
Mérieux Foundation co-organized event
8th Meeting of the GTFCC Working Group on Oral Cholera Vaccine
December 6 – 8, 2021 – Événement virtuel & Centre des Pensières, Veyrier du Lac (France)
Gavi [to 4 Dec 2021]
https://www.gavi.org/
News Releases
3 December 2021
Gavi Board approves malaria vaccine programme funding, COVAX 2022 strategic approach and measures to maintain, restore and strengthen routine immunisation
[See Week in Review above for detail]
2 December 2021
Gavi Board approves funding to support malaria vaccine roll-out in sub-Saharan Africa
2 December 2021
New Zealand kicks off 2022 COVAX fundraising efforts with NZ$ 9 million funding pledge
29 November 2021
Joint Statement on Dose Donations of COVID-19 Vaccines to African Countries
GHIT Fund [to 4 Dec 2021]
https://www.ghitfund.org/newsroom/press
GHIT was set up in 2012 with the aim of developing new tools to tackle infectious diseases that
No new digest content identified.
Global Fund [to 4 Dec 2021]
https://www.theglobalfund.org/en/news/
News & Stories
News
The Next Chapter of Our Fight Against HIV
01 December 2021 by Peter Sands, Executive Director
Current Testing Tools Uncompromised by New COVID-19 Variant of Concern Omicron (B.1.1.529)
29 November 2021
The accuracy of existing molecular (PCR, NAAT) tests appears uncompromised by COVID-19 variant Omicron (B.1.1.529), designated a Variant of Concern by the World Health Organization (WHO) on Friday. Preliminary evidence suggests that this is also true…
Global Research Collaboration for Infectious Disease Preparedness [GloPID-R] [to 4 Dec 2021]
https://www.glopid-r.org/news/
News
No new digest content identified.
Hilleman Laboratories [to 4 Dec 2021]
http://www.hillemanlabs.org/
….No “news” page or tab identified.
HHMI – Howard Hughes Medical Institute [to 4 Dec 2021]
https://www.hhmi.org/news
Press Room
No new digest content identified
Human Vaccines Project [to 4 Dec 2021]
http://www.humanvaccinesproject.org/
News
No new digest content identified
IAVI [to 4 Dec 2021]
https://www.iavi.org/newsroom
Latest News
December 3, 2021
GreenLight (ENVI) and IAVI begin work on Omicron variant-adapted COVID-19 vaccine candidate
BOSTON and NEW YORK – DECEMBER 3, 2021 – GreenLight Biosciences (ENVI) and IAVI, a non-profit scientific research organization, today announced work on a messenger RNA vaccine candidate to tackle the COVID-19 Omicron variant.
“The highly mutated Omicron variant may be partially capable of eluding existing vaccines, so rapid adaptation is an urgent priority,” said GreenLight CEO Andrey Zarur. “We have started lab work already. We will be testing a new candidate vaccine shortly in preclinical models. We have also begun an analysis on how we would proceed in clinical studies in Africa, in the context of our current clinical plans.”
IAVI President and CEO Mark Feinberg said, “While it’s impossible to say right now where Omicron first emerged, we wouldn’t have been able to respond to this threat so quickly had it not been for the skill and openness of the South African scientists who identified the variant and shared the sequence with the world. Now we must focus on bringing solutions forward both to address this new variant and the stark inequities that remain in the global availability of vaccines to prevent COVID-19.”..
November 30, 2021
GreenLight Biosciences (ENVI) partners with IAVI to accelerate COVID-19 vaccine trial in Africa
IAVI’s extensive clinical trial experience will be combined with GreenLight COVID-19 vaccine candidate.
Goal is to broaden and accelerate access to messenger RNA (mRNA) COVID-19 vaccines.
Phase I trial planned for late Q1 2022.
Plan speeds creation of vaccines in Africa, for Africa, and suitable for use in low-income countries globally.
International Coalition of Medicines Regulatory Authorities [ICMRA]
http://www.icmra.info/drupal/en/news
Selected Statements, Press Releases, Research
No new digest content identified.
ICRC [to 4 Dec 2021]
https://www.icrc.org/en/whats-new
Selected News Releases, Statements, Reports
ICRC: Omicron highlights the need to step up global vaccinations, including in conflict zones
Statement from Esperanza Martinez, the head of the COVID-19 Crisis Management Team at the ICRC on vaccine inequity.
02-12-2021 | News release
IFFIm
http://www.iffim.org/
Press Releases/Announcements
No new digest content identified.
IFRC [to 4 Dec 2021]
http://media.ifrc.org/ifrc/news/press-releases/
Selected Press Releases, Announcements
02/12/2021
“Impulsive reactions are an affront to the global solidarity we need to successfully respond to a crisis of this scale.” [WHA]
29/11/2021
“Impulsive and ineffective reactions” to COVID-19’s Omicron variant will send more Africans into poverty
Mohammed Mukhier, IFRC Regional Director for Africa
29/11/2021
“The pandemic has both thrived on inequities and exacerbated them”
Institut Pasteur [to 4 Dec 2021]
https://www.pasteur.fr/en/press-area
Press Documents
Press release
26.11.2021
ComCor study: new results on places of infection with SARS-CoV-2 and analysis of the efficacy of messenger RNA vaccines against the Delta variant
From May 23 to August 13, 2021, the Institut Pasteur, in partnership with the French National Health Insurance Fund (CNAM), Santé publique France and the Ipsos institute, conducted the fourth part of the ComCor epidemiological study on circumstances and places of infection with the SARS-CoV-2 virus in France. The aim of the study was to identify the socio-demographic factors, places visited and behaviors associated with a higher risk of infection with SARS-CoV-2. The study was also used to carry out a real-world analysis of the effect of previous infection with COVID-19 and the efficacy of messenger RNA vaccines against symptomatic infection with the Delta variant. The findings were published in The Lancet Regional Health Europe on November 26, 2021.
IOM / International Organization for Migration [to 4 Dec 2021]
http://www.iom.int/press-room/press-releases
News – Selected
News
01 Dec 2021
IOM’s World Migration Report Shows Global Displacement Rising Despite COVID-19 Mobility Limits
ISC / International Science Council [to 4 Dec 2021]
https://council.science/current/
ISC is a non-governmental organization with a unique global membership that brings together 40 international scientific Unions and Associations and over 140 national and regional scientific organizations including Academies and Research Councils.
News
Press releases
The International Year of Basic Sciences for Sustainable Development proclaimed by the United Nations General Assembly for 2022
03.12.2021
We need more basic sciences to achieve Agenda 2030 and its 17 Sustainable Development Goals. This is the message sent to the world by the United Nations General Assembly on 2 December 2021: Member States approved by consensus the resolution 76/A/L.12 promulgating the year 2022 as the International Year of Basic Sciences for Sustainable Development (IYBSSD2022).
With this resolution, the United Nations General Assembly ‘invites all [its] Member States, organizations of the United Nations system and other global, regional and subregional organizations, as well as other relevant stakeholders, including academia, civil society, inter alia, international and national non-governmental organizations, individuals and the private sector, to observe and raise awareness of the importance of basic sciences for sustainable development, in accordance with national priorities’…
IVAC [to 4 Dec 2021]
https://www.jhsph.edu/research/centers-and-institutes/ivac/index.html
Updates; Events
No new digest content identified.
IVI [to 4 Dec 2021]
http://www.ivi.int/
IVI News & Announcements
No new digest content identified.
Johns Hopkins Center for Health Security [to 4 Dec 2021]
https://www.centerforhealthsecurity.org/news/center-news/
Center News
No new digest content identified.
MSF/Médecins Sans Frontières [to 4 Dec 2021]
http://www.msf.org/
Latest [Selected Announcements]
Ukraine
Volunteers help fellow villagers access healthcare in conflict-affected eastern Ukraine
Project Update 3 Dec 2021
Coronavirus COVID-19 pandemic
In an unequal world, our response to COVID-19 cannot be one size-fits-all
Opinion 3 Dec 2021
Myanmar
Myanmar: Political turmoil threatens HIV care
MSF Asia Pacific. 1 Dec 2021
HIV/AIDS
DRC: People are still dying unnecessarily from HIV
Project Update 1 Dec 2021
World AIDS Day
Why are people living with HIV still dying of AIDS?
Opinion 1 Dec 2021
World Health Assembly
Pandemic preparedness and response: some lessons learnt
Statement 30 Nov 2021
MSF delivered a statement on 30 November 2021 during the World Health Assembly Special Session.
National Academy of Medicine – USA [to 4 Dec 2021]
https://nam.edu/programs/
Selected News/Programs/Events
Expert Paper from the National Academy of Medicine Identifies Lessons Learned and Compelling Needs to Center Patients, Families, and Communities in the Delivery of Health and Health Care After COVID-19
November 29, 2021
WASHINGTON – An individually-authored discussion paper was published in NAM Perspectives today that focuses on the experience and needs of patients, families, and communities, was published on November 29, 2021. Patients, Families, and Communities COVID-19 Impact Assessment: Lessons Learned and Compelling Needs reviews the impact and implications of COVID-19 on patients, families, and communities, offers […]
National Academy of Sciences – USA [to 4 Dec 2021]
http://www.nasonline.org/news-and-multimedia/
News
No new digest content identified.
National Vaccine Program Office – U.S. HHS [to 4 Dec 2021]
https://www.hhs.gov/vaccines/about/index.html
Upcoming Meetings/Latest Updates
No new digest content identified.
NIH [to 4 Dec 2021]
http://www.nih.gov/news-events/news-releases
News Releases
NIH Statement on World AIDS Day 2021
December 1, 2021 — Statement of Anthony S. Fauci, M.D. and Maureen M. Goodenow, Ph.D.
… Although the HIV vaccine field has been marked by disappointing results over the years, finding a safe, effective and durable HIV vaccine remains an NIH priority. Currently, the Phase 3 Mosaico/HPTN 706 HIV vaccine clinical trial is underway in the Americas and Europe with results expected in 2024. Lessons learned from highly effective SARS-CoV-2 vaccines also offer an encouraging path forward for HIV vaccine discovery by providing applications for new vaccine platforms, such as mRNA, and novel strategies for rapidly identifying vaccine targets. Additionally, promising outcomes utilizing bNAbs suggest it may be possible to achieve an HIV vaccine with a high level of efficacy—an almost inconceivable scientific possibility several years ago…
Too many people with HIV fail to achieve durable viral suppression
November 30, 2021 — NIH-funded study estimates global progress toward UNAIDS goal.
Article: WM Han et al. Global estimates of viral suppression in children and adolescents and adults on antiretroviral therapy adjusted for missing viral load measurements: a multiregional, retrospective cohort study in 31 countries(link is external). The Lancet HIV (2021).
OECD [to 4 Dec 2021]
http://www.oecd.org/newsroom/publicationsdocuments/bydate/
Newsroom
Japan: broaden the digital transition to strengthen economic recovery from COVID-19, says OECD
3-December-2021
Rising vaccination rates and a rebound in exports are helping Japan’s economy to recover from the shock caused by COVID-19, although challenges remain. Investing in technology, education and professional training to broaden and accelerate the country’s digital transformation would help to spur productivity growth and reinforce public finances, according to a new OECD report.
PATH [to 4 Dec 2021]
https://www.path.org/media-center/
Press Releases
PATH welcomes commitment by Gavi board to finance wider rollout of the world’s first malaria vaccine, RTS,S
December 2, 2021 by PATH
Today’s important decision enables low- and middle-income countries to consider adding RTS,S as an additional tool to combat malaria
Additional investments will be needed to expand vaccine supply if current demand projections are realized
Together with partners and other stakeholders, PATH is working to help ensure sufficient malaria vaccine supply to meet potential demand
Sabin Vaccine Institute [to 4 Dec 2021]
http://www.sabin.org/updates/pressreleases
Statements and Press Releases
No new digest content identified.
UNAIDS [to 4 Dec 2021]
http://www.unaids.org/en
Selected Press Releases/Reports/Statements
3 December 2021
Strengthening Haiti’s HIV response through community-led monitoring
2 December 2021
Community health workers strengthen HIV and COVID-19 responses
1 December 2021
Letter from Prince Harry to Dr Tedros Adhanom Ghebreyesus and Ms Winnie Byanyima on World AIDS Day
UNHCR Office of the United Nations High Commissioner for Refugees [to 4 Dec 2021]
http://www.unhcr.org/en-us/media-centre.htmlS
Selected News Releases, Announcements
Prolonged COVID-19 pandemic deepens hardship for over 12 million forcibly displaced people with disabilities
3 Dec 2021
At least 12 million people with disabilities are forcibly displaced worldwide, UNHCR, the UN Refugee Agency, estimates, and their already precarious situation is becoming harder as the COVID-19 pandemic drags on.
“Forced displacement disproportionately affects people with disabilities. They are often at higher risk of violence, discrimination, neglect, gender-based violence, exploitation and abuse, face barriers to access basic services, and are often excluded from education and livelihood opportunities,” said Gillian Triggs, UNHCR’s Assistant High Commissioner for Protection.
“While many refugees faced these unacceptable, pre-existing risks of exclusion and discrimination, we fear that the prolonged COVID-19 pandemic is only deepening inequalities and hardship,” she added.
While estimates say over 12 million people with disabilities have been forcibly displaced by persecution, violence and human rights violations worldwide, surveys and assessments suggest the real number may be much higher.
Challenges and risks begin at the moment of flight. In sudden onset disasters, abrupt violence, and active hostilities, people with disabilities are often left behind by family members due to the sudden nature of armed attacks; in extreme cases reported, people were abandoned while chained.
On reaching safety, services and facilities, including assistance programmes and protection, may be inaccessible. People with disabilities may face physical barriers and obstacles to reach, enter or use basic services, or key information may be delivered in formats not accessible to them.
Refugees and internally displaced and stateless people with disabilities were already less likely to access health care, education and employment opportunities, and the global crisis has further compounded this situation.
On the International Day of Persons with Disabilities today, UNHCR is urging national authorities to do more to secure the rights of forcibly displaced and stateless people with disabilities and to counter all forms of discrimination…
UNICEF [to 4 Dec 2021]
https://www.unicef.org/media/press-releases
Press Releases, News Notes, Statements [Selected]
Press release
11/29/2021
Joint Statement on Dose Donations of COVID-19 Vaccines to African Countries
A joint statement from the African Vaccine Acquisition Trust (AVAT), the Africa Centres for Disease Control and Prevention (Africa CDC) and COVAX
[See Milestones above for detail]
Press release
11/29/2021
A child was infected with HIV every two minutes in 2020 – UNICEF
A prolonged COVID-19 pandemic is deepening the inequalities that have long driven the HIV epidemic, UNICEF warns Ahead of World AIDS Day.
Unitaid [to 4 Dec 2021]
https://unitaid.org/
Featured News
30 November 2021
COVID-19 creates new urgency for access to HIV self-testing, says global health agency Unitaid
The number of countries with self-testing policies in place has increased by nearly 15-fold since 2015, with an estimated 192 million self-tests needed to cover low- and middle-income countries by 2025.
Geneva – Disruptions and delays to HIV services caused by the COVID-19 pandemic led to declines in HIV testing and diagnoses in 2020 for the first time in more than two decades. HIV self-testing, which has contributed to a 40% reduction in the number of people who do not know their HIV status since 2015, is now crucial to maintaining access to HIV care for millions of people in the face of new COVID-19 roadblocks.
With more than 37 million people living with HIV worldwide – an estimated 6.1 million of whom do not know their status – receiving a diagnosis is a vital first step in accessing treatment.
Unitaid has led a large-scale effort to create access to HIV treatment and prevention through self-testing, with more than US$100 million invested since 2015. This work has helped reduce prices for self-tests, generate demand, demonstrate implementation pathways, and support scale up across 14 countries in Africa[1], with more recent work in India and Indonesia looking to further expand successful self-testing strategies to more people…
While procurement of HIV self-tests is rising, it is greatly outpaced by the need. By 2025, the number of self-tests procured is projected to fill just 15% of the estimated 192 million tests needed in low- and middle-income countries. And although the funding allocated for self-testing has increased significantly over the past several years, an additional US$104 million is needed to cover the costs of the 29 million self-tests expected to be procured.
Ahead of World AIDS Day, 1 December, Unitaid is calling for urgent funding and scale-up of HIV self-testing to protect progress threatened by COVID-19 and secure pathways to HIV treatment and prevention services for millions of people worldwide.
Vaccine Equity Cooperative [nee Initiative] [to 4 Dec 2021]
https://vaccineequitycooperative.org/news/
News
No new digest content identified.
Vaccination Acceptance & Demand Initiative [Sabin) [to 4 Dec 2021]
https://www.vaccineacceptance.org/
Announcements
No new digest content identified.
Vaccine Confidence Project [to 4 Dec 2021]
http://www.vaccineconfidence.org/
News, Research and Reports
No new digest content identified.
Vaccine Education Center – Children’s Hospital of Philadelphia [to 4 Dec 2021]
http://www.chop.edu/centers-programs/vaccine-education-center
News
No new digest content identified.
Wellcome Trust [to 4 Dec 2021]
https://wellcome.ac.uk/news
News. Opinion, Reports
News
“There isn’t a united global strategy” – we need to ensure vaccine equity to end Covid-19
30 November 2021
As the World Health Organization (WHO) announces a new variant of concern, we find out what people around the world think about Covid-19 vaccine distribution.
News
Public trust in scientists rose during the Covid-19 pandemic
29 November 2021
Insights from the Wellcome Global Monitor – the largest global survey of how people think and feel about science – found levels of trust in scientists have increased since 2018, placing it on par with doctors and nurses…
Key findings
[1] Covid-19 has put science in the public eye like never before. The Wellcome Global Monitor offers insight into how people think and feel about science and major health challenges worldwide.
Key findings include:
[2] The impact of Covid-19 has been uneven around the world. The Wellcome Global Monitor found that the pandemic has had a disproportionate impact on low-income countries and people with low incomes across all countries.
[3] People’s trust in science and scientists has increased. Globally, those who said they trust scientists ‘a lot’ rose from 34% in 2018 to 43% by the end of 2020. These increases were highest among those who indicated they know ‘some’ or ‘not much/nothing at all’ about science.
People are not sure that their governments value scientific advice. World leaders need to listen and show that they share public confidence in the role of science in solving health challenges fairly for everyone.
The Wistar Institute [to 4 Dec 2021]
https://www.wistar.org/news/press-releases
Press Releases
Dec. 2, 2021
Fox Chase Cancer Center, The Wistar Institute Announce Opening of Phase 1 Clinical Trial of Anticancer Drug Candidate
PHILADELPHIA — (Dec. 2, 2021) — Fox Chase Cancer Center has opened an investigator-initiated, phase 1 clinical trial to evaluate the safety and efficacy of gamitrinib in patients with advanced cancer. Gamitrinib is a first-in-class, mitochondrial-targeted inhibitor of the molecular chaperone heat shock protein-90 (HSP90) that works by inhibiting tumor cell metabolism and survival.
Press Release
Nov. 30, 2021
Wistar Scientists Identify Genes Critical to Protecting Ovarian Cancer from the Immune System
Discovery could make immunotherapy more effective for ovarian and other cancers.
WFPHA: World Federation of Public Health Associations [to 4 Dec 2021]
https://www.wfpha.org/
Latest News – Blog
A Vaccine TRIPS Waiver Now: Letter to WTO
Nov 29, 2021
Vaccination can save lives in millions. Massive upscaling of vaccine production is required across the globe with the COVID-19 pandemic continuing and with the prospect of new waves, and new variants in the future.
The World Federation of Public Health Associations, the Sustainable Health Equity Movement and the Global Network for Academic Public Health urge the World Trade Organisation to do everything in its power to achieve agreement to a temporary vaccine Trade-related Intellectual Property Rights waiver at its Ministerial Conference.
We believe the vaccine TRIPS waiver is the single most important action that can be taken to secure full and fair access to vaccine across the world, to give us the best possible chance of ending this pandemic, saving lives, and preventing disease and disability.
Letter
World Bank [to 4 Dec 2021]
http://www.worldbank.org/en/news/all
Selected News, Announcements
An Uneven Recovery: the Impact of COVID-19 on Latin America and the Caribbean
WASHINGTON D.C., November 29, 2021– Employment rates in some Latin American and Caribbean countries have experienced a relative recovery, although in most, rates fall short of pre-pandemic levels. The…
Date: November 29, 2021 Type: Press Release
World Organisation for Animal Health (OIE) [to 4 Dec 2021]
https://www.oie.int/en/media/news/
Press Releases, Statements
Tripartite and UNEP support OHHLEP’s definition of “One Health”
Joint Tripartite (FAO, OIE, WHO) and UNEP Statement
1 Dec 2021
The Food and Agriculture Organization of the United Nations (FAO), the World Organisation for Animal Health (OIE), the United Nations Environment Programme (UNEP) and the World Health Organization (WHO) welcome the newly formed operational definition of One Health from their advisory panel, the One Health High Level Expert Panel (OHHLEP), whose members represent a broad range of disciplines in science and policy-related sectors relevant to One Health from around the world. The four organizations are working together to mainstream One Health so that they are better prepared to prevent, predict, detect, and respond to global health threats and promote sustainable development.
The One Health definition developed by the OHHLEP states:
“One Health is an integrated, unifying approach that aims to sustainably balance and optimize the health of people, animals and ecosystems. It recognizes the health of humans, domestic and wild animals, plants, and the wider environment (including ecosystems) are closely linked and inter-dependent.The approach mobilizes multiple sectors, disciplines and communities at varying levels of society to work together to foster well-being and tackle threats to health and ecosystems, while addressing the collective need for clean water, energy and air, safe and nutritious food, taking action on climate change, and contributing to sustainable development.”
WTO – World Trade Organisation [to 4 Dec 2021]
http://www.wto.org/english/news_e/news_e.htm
WTO News and Events
Negotiations on services domestic regulation conclude successfully in Geneva
2 December 2021
Heads of Geneva delegations from the 67 WTO members participating in the Joint Initiative on Services Domestic Regulation announced on 2 December the successful conclusion of negotiations aimed at slashing administrative costs and creating a more transparent operating environment for service providers hoping to do business in foreign markets.
DG calls on members to agree on pandemic response, fisheries subsidies by end-February
2 December 2021
Following last week’s eleventh-hour decision by WTO members to postpone the 12th Ministerial Conference, negotiations are continuing in Geneva, and Director-General Ngozi Okonjo-Iweala today (2 December) urged delegation heads to redouble their efforts to bridge differences. She called on them to conclude agreements on the WTO system’s response to pandemics as well as on curbing harmful fisheries subsidies by the end of February 2022 to pave the way for approval by ministers. “Seven billion people are waiting for us on TRIPS and pandemic response. And 260 million people are waiting for us on fisheries subsidies,” she said.
[See Milestones above for detail]
::::::
ARM [Alliance for Regenerative Medicine] [to 4 Dec 2021]
https://alliancerm.org/press-releases/
Selected Press Releases
No new digest content identified.
BIO [to 4 Dec 2021]
https://www.bio.org/press-releases
Press Releases, Letters, Testimony, Comments [Selected]
No new digest content identified.
DCVMN – Developing Country Vaccine Manufacturers Network [to 4 Dec 2021]
http://www.dcvmn.org/
News; Upcoming events
No new digest content identified.
ICBA – International Council of Biotechnology Associations [to 4 Dec 2021]
https://internationalbiotech.org/news/
News
No new digest content identified.
IFPMA [to 4 Dec 2021]
http://www.ifpma.org/resources/news-releases/
Selected Press Releases, Statements, Publications
Global pharmaceutical industry leaders meet with UK Prime Minister to discuss leadership in global health
London, 3 December, 2021 – Yesterday, global leaders from the pharmaceutical industry met with the Prime Minister, senior UK Cabinet Ministers and the NHS Chief Executive, to discuss global health and innovation. Their discussions focused on how the industry and UK Government can work together to drive pharmaceutical innovation and rapidly deliver the next generation of medicines to patients worldwide, and deliver on the ambition of the UK Government’s Life Sciences Vision…
Global biopharmaceutical industry early response to Omicron
3 December 2021 – COVID-19, the worst pandemic the world has seen in 100 years, has shown us that we cannot let our guard down. The global biopharmaceutical industry understands the concern caused by the emergence of the Omicron variant of COVID-19 since it was declared a variant of concern by the World Health Organization.
As a science-driven industry addressing some of the world’s biggest healthcare challenges, the biopharmaceutical industry has demonstrated over the last two years how it is uniquely positioned to respond rapidly to COVID-19. Biopharmaceutical companies are bringing the same commitment to innovation in responding to the Omicron variant.
It is early days in terms of understanding Omicron. Together with the broader scientific community, public health and regulatory bodies around the world, our industry will again bring its experience and expertise to bear on new variants such as Omicron.
None of this work would have been possible if the South African and Botswana scientists had not shared the Omicron variant so swiftly through tried and tested platforms such as GISAID. Their fast sharing of the data has bought us all time in responding to and potentially controlling this new variant. The current situation reflects the advantage of fast and effective sharing of harmful pathogens and variants, which allows us to develop effective vaccines, treatments, and diagnostics.
Biopharmaceutical companies have hit the ground running in response to the Omicron variant, as they have done with previous variants (Beta and Delta). Crucially, they are looking at how both authorized and unapproved vaccines and treatments can stand up to this latest variant. A number of vaccine developers have already stated that they will develop Omicron targeting candidates.
The emergence of Omicron variant comes on top of a surge in COVID-19 cases in many regions of the world, caused by the dominant Delta variant at a time when health systems are already working hard to cope with seasonal endemic infections. Reducing the toll of the pandemic on lives and livelihoods requires not only continued innovation in the development and production of vaccines, treatments, and diagnostics, but also urgently addressing inequality in vaccine distribution and country readiness for vaccination. Our Five Steps to urgently advance COVID-19 vaccine equity announced in May 2022 are as relevant and as demanding of action today as they were when they were first launched.
Joint Statement on Patient Solidarity Day
Geneva, December 3rd, 2021 – On Patient Solidarity Day, the International Alliance of Patients’ Organizations (IAPO), the International Pharmaceutical Federation (FIP), the International Federation of Pharmaceutical Manufacturers and Associations (IFPMA), the International Hospital Federation (IHF) and the International Council of Nurses (ICN), join patients and families all over the world, in calling for governments and all health stakeholders to collaborate in the implementation of the WHO Flagship Global Patient Safety Action Plan 2021-2030 (GPSAP 2021-30) that was adopted at the 74th World Health Assembly this year.
Every year, large numbers of patients are harmed or die because of unsafe health care, creating a high burden of death and disability worldwide, especially in low- and middle-income countries. On average, an estimated one in 10 patients is subject to an adverse event while receiving hospital care in high-income countries. Available evidence suggests that 134 million adverse events due to unsafe care occur in hospitals in low- and middle-income countries, contributing to around 2.6 million deaths every year. According to recent estimates, the social cost of patient harm can be valued at US$ 1 trillion to 2 trillion a year.[1]
Patient safety is fundamental to the provision of health care in all settings. However, avoidable adverse events, errors and risks associated with health care remain major challenges for patient safety globally. The global COVID-19 pandemic has highlighted the importance of building a culture of trust in healthcare systems, health professionals and medical products. Trust can be undermined by products and interventions that do not adhere to the highest standards of quality and ethical standards.
We therefore call on all governments and health stakeholders to deploy patient safety as a health priority in health sector policies and programmes to achieve universal health coverage; and for all health stakeholders to uphold the guiding principles of GPSAP 2021-30, by working to:
Implement policies to eliminate avoidable harm in health
Foster high-reliability systems
Ensure safety of clinical processes
Maintain patient and family engagement
Secure continuous health worker education, skills and safety
Today, representing key actors in health systems, we reaffirm our commitment to provide the highest quality health care and health products to patients and to work towards the GPSAP 2021-30 vision of “a world in which no one is harmed in health care, and every patient receives safe and respectful care, every time, everywhere”.
[1] Global Patient Safety Action Plan 2021–2030 Towards eliminating avoidable harm in health care
Biopharmaceutical industry collaborates on the implementation of the G7 100 Days Mission to improve readiness
Published on: 03 December 2021
The biopharmaceutical industry welcomes the publication of the 100 Days Mission – First Implementation Report released on 2 December, which maps out key actions needed from all stakeholders to build global pandemic preparedness. As one of the implementation partners, we welcome the strong sense of public-private work involved to achieve this shared ambition is important and will be critical to future success.
The industry has played a full and integral role in tackling COVID-19 and we are committed to working with all partners – including the German G7 Presidency – to ensure the world is better prepared against future pandemics.
Following the World Health Assembly’s decision this week to launch a process to seek a global agreement on pandemic prevention, preparedness and response and we also support working with governments, international organizations and other stakeholders to make sure that policies and plans are developed in a way that achieves the effective response the world requires to tackle any future pandemic.
The 100 Day Mission roadmap indicates that it is well underway towards completion. It should provide useful insights for the global pandemic preparedness process that will be negotiated over the coming years. Both processes should recognise that emerging pathogens must be shared on or before Day 0 of future pandemics, and that any delays due to conditions applied to such sharing must be avoided if we are to achieve the 100 days objectives.
As a global community, we applaud the speed with which scientists are currently sharing pathogens. Their efforts should not be restricted by unclear legislation or unnecessary paperwork.
From the very beginning of the pandemic the biopharmaceutical industry has worked in global partnerships with governments, health systems and across industry to deliver safe and effective COVID-19 vaccines and treatments.
We must now build on the successes of COVID-19, exploring new public-private partnerships to accelerate vaccine and drug discovery for priority pathogens and prepare for new pathogens.
COVID-19 has clearly demonstrated the importance of investment into R&D against future pathogens and COVID variants. These investments from across the R&D ecosystem – including large biopharmaceutical companies, small biotechs and academic researchers – is completely dependent on strong protection of intellectual property.
International Generic and Biosimilar Medicines Association [IGBA]
https://www.igbamedicines.org/
News
No new digest content identified.
International Alliance of Patients’ Organizations – IAPO [to 4 Dec 2021]
https://www.iapo.org.uk/news/topic/6
Press and media [Selected]
Joint Statement On Patient Solidarity Day 2021
London, December 3rd, 2021 – On Patient Solidarity Day, the International Alliance of Patients’ Organizations (IAPO), the International Pharmaceutical Federation (FIP), the International Federation of Pharmaceutical Manufacturers and Associations (IFPMA) and the International Hospital…
Over 3000 patient advocates in Asia-Pacific come together calling for recovering together through compassion, insight and co-creation
On 16 and 17 November 2021, 3800 patient advocates, government and policy makers, industry representatives, healthcare professionals, academia representatives, researchers and media came together for the virtual 3rd Asia-Pacific Patients Congress (APPC 2021) to share their vision and lived…
PhRMA [to 4 Dec 2021]
http://www.phrma.org/
Latest News [Selected]
No new digest content identified.