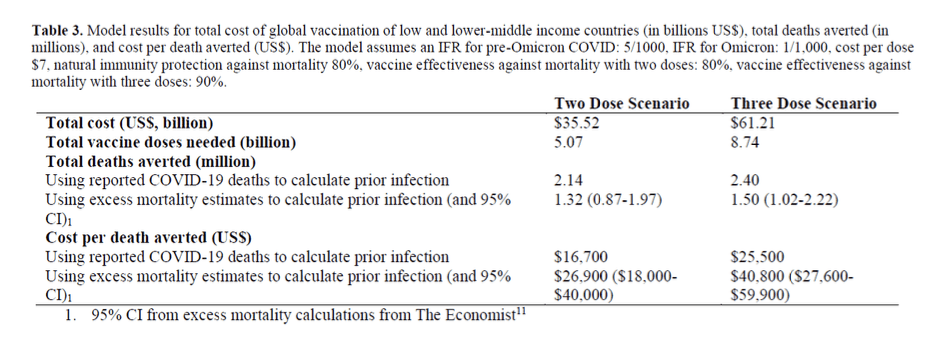
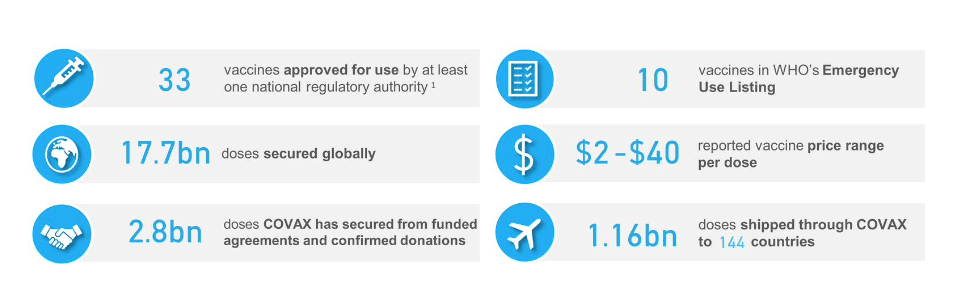
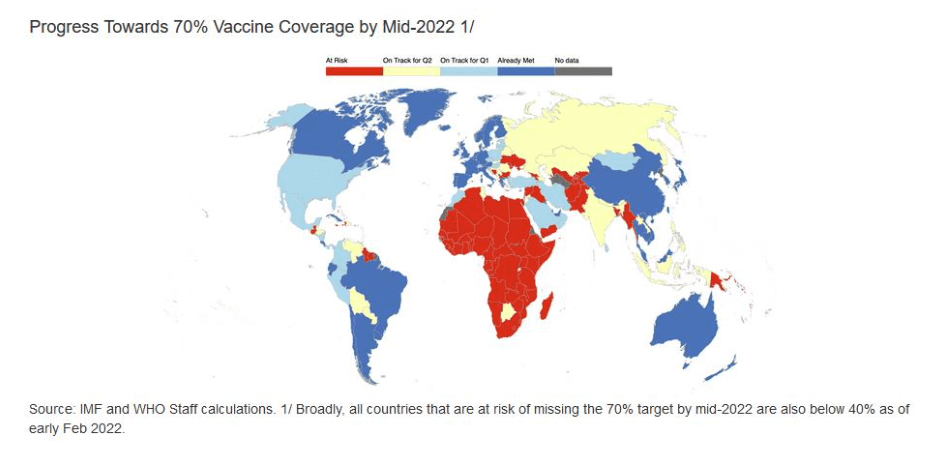
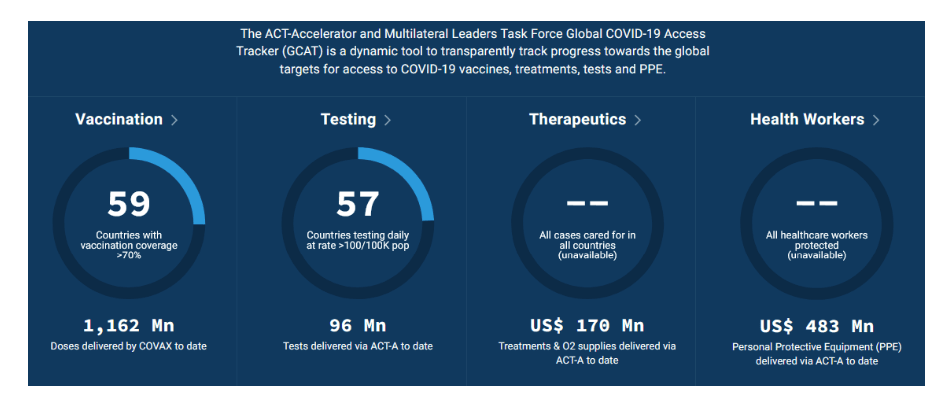
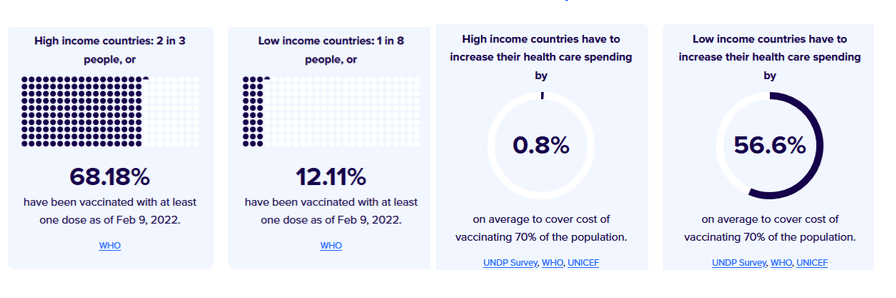
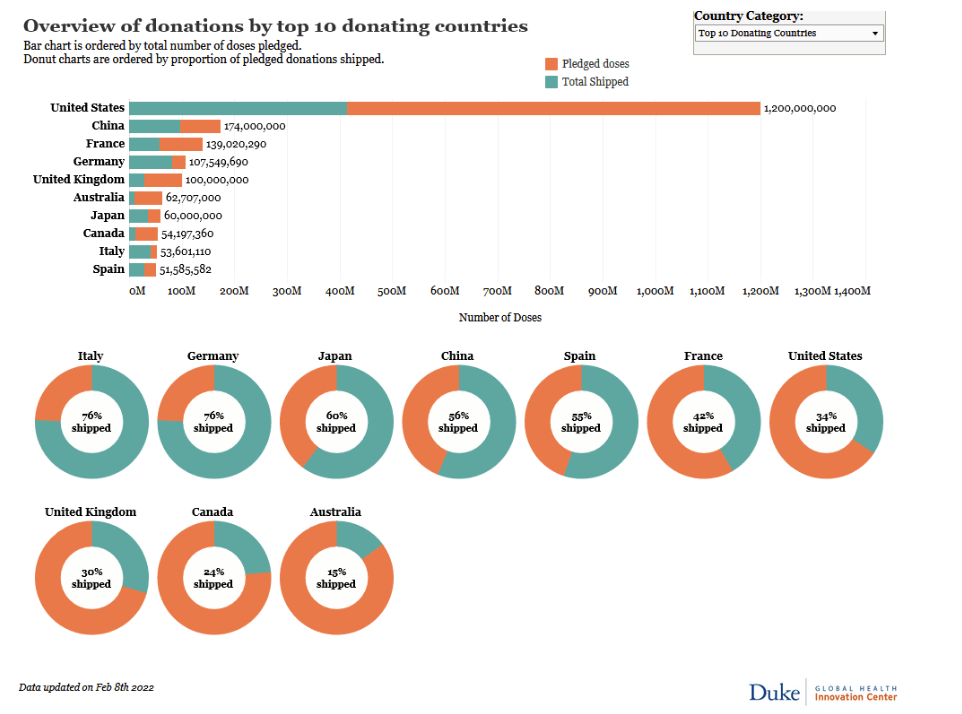
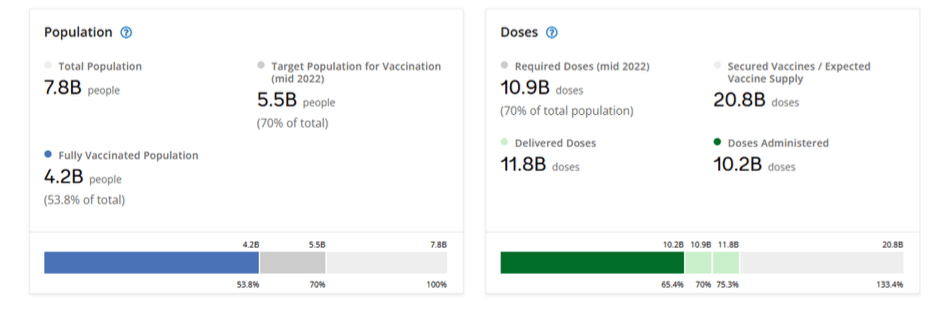
Pre-Print Servers
Gates Open Research
https://gatesopenresearch.org/browse/articles
[Accessed 19 Feb 2022]
Research Article metrics
Revised
Setting up child health and mortality prevention surveillance in Ethiopia [version 2; peer review: 1 approved, 1 approved with reservations]
Anna C. Seale, Nega Assefa, Lola Madrid, Stefanie Wittmann, Hanan Abdurahman, Nardos Teferi, Letta Gedefa, Alexander Mohamed, Natnael Debela, Tseyon Tesfaye, Tigistu Samuel, Mehret Dubale, Hiwot Yigzaw, Eyoel Taye, Workalemahu Bekele, Caroline Ackley, Gutema Imana Keno, Yosef Zegeye, Zerihun Girma, Ketema Degefa, Berhanu Damisse, Adugna Tadesse, Mohammed Aliyi, Gurmu Feyissa, Yenenesh Tilahun, Getahun Wakwaya, Bizunesh Sintayehu, Getamesay Abayneh, Addisu Alemu, Emmanuel Azore, Joe Oundo, Zelalem T Mariam, Dadi Marami, Mulu Berihun, Mussie Berhanu, Mahlet Mekonnen, Andualem Alemayehu, Nana Sarkodie-Mensah, Shirine Voller, Boniface Jibendi, Abraham Aseffa, Taye Balcha, Robert F. Breiman, Scott Dowell, Asnake Worku, Tsigereda Kifle, Ebba Abate, Yadeta Dessie, J. Anthony G. Scott
Peer Reviewers Godfrey M. Bigogo; Bernt Lindtjørn
Funders
Bill and Melinda Gates Foundation
Wellcome
LATEST VERSION PUBLISHED 14 Feb 2022
medRxiv
https://www.medrxiv.org/content/about-medrxiv
medRxiv is a free online archive and distribution server for complete but unpublished manuscripts (preprints) in the medical, clinical, and related health sciences. Preprints are preliminary reports of work that have not been certified by peer review. They should not be relied on to guide clinical practice or health-related behavior and should not be reported in news media as established information. medRxiv is for the distribution of preprints – complete but unpublished manuscripts – that describe human health research conducted, analyzed, and interpreted according to scientific principles…
Adolescent vaccination with BNT162b2 (Comirnaty, Pfizer-BioNTech) vaccine and effectiveness against COVID-19: national test-negative case-control study, England
Annabel A Powell, Freja Kirsebom, Julia Stowe, Kelsey McOwat, Vanessa Saliba, Mary E Ramsay, Jamie Lopez Bernal, Nick Andrews, Shamez N Ladhani
medRxiv 2021.12.10.21267408; doi: https://doi.org/10.1101/2021.12.10.21267408
Meta-analyses on SARS-CoV-2 Viral Titers in Wastewater and Their Correlations to Epidemiological Indicators
David Mantilla-Calderon, Kaiyu (Kevin) Huang, Aojie Li, Kaseba Chibwe, Xiaoqian Yu, Yinyin Ye, Lei Liu, Fangqiong Ling
medRxiv 2022.02.14.22270937; doi: https://doi.org/10.1101/2022.02.14.22270937
City-wide wastewater genomic surveillance through the successive emergence of SARS-CoV-2 Alpha and Delta variants
F.S. Brunner, M.R. Brown, I. Bassano, H. Denise, M.S. Khalifa, M. Wade, J.L. Kevill, D.L. Jones, K. Farkas, A.R. Jeffries, The COVID-19 Genomics UK (COG-UK) Consortium, E. Cairns, C. Wierzbicki, S. Paterson
medRxiv 2022.02.16.22269810; doi: https://doi.org/10.1101/2022.02.16.22269810
Racial discrimination, low trust in the health system, and COVID-19 vaccine uptake: a longitudinal observational study of 633 UK adults from ethnic minority groups
Elise Paul, Daisy Fancourt, Mohammad Razai
medRxiv 2021.08.26.21262655; doi: https://doi.org/10.1101/2021.08.26.21262655
SARS-CoV-2 infection in Africa: A systematic review and meta-analysis of standardised seroprevalence studies, from January 2020 to December 2021
HC Lewis, H Ware, M Whelan, L Subissi, Z Li, X Ma, A Nardone, M Valenciano, B Cheng, K Noel, C Cao, M Yanes-Lane, B Herring, A Talisuna, N Nsenga, T Balde, DA Clifton, M Van Kerkhove, DL Buckeridge, N Bobrovitz, J Okeibunor, RK Arora, I Bergeri, the UNITY Studies Collaborator Group
medRxiv 2022.02.14.22270934; doi: https://doi.org/10.1101/2022.02.14.22270934
Assessing the Profile of Unvaccinated COVID-19 Individuals in African American and Latinx Communities in Eastern Pennsylvania
Kenya M. Colvin, Kennedy S. Camara, Latasha S. Adams, Adline P. Sarpong, Danielle G. Fuller, Sadie E. Peck, Anthony S. Ramos, Ariana L. Acevedo, Meless A. Badume, Shae-lyn A. Briggs, Tiffany N. Chukwurah, Zanett Davila-Gutierrez, James A. Ewing, Jemimah O. Frempong, Amirah A. Garrett, Steven J. Grampp, Jahasia W. Gillespie, Emmanuel J. Herrera, Emis J. Maddox, John C. Pelaez, Olivia L. Quartey, Fanny Rodriguez, Luis A. Vasquez, Brian J. Piper, Swathi Gowtham
medRxiv 2022.02.11.22270504; doi: https://doi.org/10.1101/2022.02.11.22270504
Co-infection with SARS-COV-2 Omicron and Delta Variants Revealed by Genomic Surveillance
Rebecca J Rockett, Jenny Draper, Mailie Gall, Eby M Sim, Alicia Arnott, Jessica E Agius, Jessica Johnson-Mackinnon, Elena Martinez, Alexander P Drew, Clement Lee, Christine Ngo, Marc Ramsperger, Andrew N Ginn, Qinning Wang, Michael Fennell, Danny Ko, Linda Huston, Lukas Kairaitis, Edward C Holmes, Matthew N O’Sullivan, Sharon C-A Chen, Jen Kok, Dominic E Dwyer, Vitali Sintchenko
medRxiv 2022.02.13.22270755; doi: https://doi.org/10.1101/2022.02.13.22270755
Unpacking COVID-19 and Conspiracy Theories in the UK Black Community
Tushna Vandrevala, Jane Hendy, Kristin Hanson, Lailah Alidu, Aftab Ala
medRxiv 2022.02.12.22270438; doi: https://doi.org/10.1101/2022.02.12.22270438
Wellcome Open Research [to 19 Feb 2022]
https://wellcomeopenresearch.org/browse/articles
[Accessed 19 Feb 2022]
Wellcome Open Research provides all Wellcome researchers with a place to rapidly publish any results they think are worth sharing. All articles benefit from rapid publication, transparent peer review and editorial guidance on making all source data openly available.
Research Article metrics
Revised
Date of introduction and epidemiologic patterns of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in Mogadishu, Somalia: estimates from transmission modelling of satellite-based excess mortality data in 2020 [version 2; peer review: 1 approved, 1 approved with reservations]
Mihaly Koltai, Abdihamid Warsame, Farah Bashiir, Terri Freemantle, Chris Reeve, Chris Williams, Mark Jit, Stefan Flasche, Nicholas G. Davies, CMMID COVID-19 working group, Ahmed Aweis, Mohamed Ahmed, Abdirisak Dalmar, Francesco Checchi
Peer Reviewers Zindoga Mukandavire; Bhaswar Ghosh
Funders
Wellcome Trust
Foreign, Commonwealth and Development Office
UK Research and Innovation
LATEST VERSION PUBLISHED 18 Feb 2022
Systematic Review metrics AWAITING PEER REVIEW
Influences on User Trust in Healthcare Artificial Intelligence: A Systematic Review [version 1; peer review: awaiting peer review]
Eva Jermutus, Dylan Kneale, James Thomas, Susan Michie
Peer Reviewers Invited
Funders
Wellcome Trust
Economic and Social Research Council
PUBLISHED 18 Feb 2022
Research Article metrics
Revised
Lessons learned and lessons missed: impact of the coronavirus disease 2019 (COVID-19) pandemic on all-cause mortality in 40 industrialised countries and US states prior to mass vaccination [version 2; peer review: 1 approved, 1 approved with reservations]
Vasilis Kontis, James E. Bennett, Robbie M. Parks, Theo Rashid, Jonathan Pearson-Stuttard, Perviz Asaria, Bin Zhou, Michel Guillot, Colin D. Mathers, Young-Ho Khang, Martin McKee, Majid Ezzati
Peer Reviewers Rajeev Gupta; Virgilio Gómez-Rubio
Funders
Wellcome Trust
British Heart Foundation
US Environmental Protection Agency
LATEST VERSION PUBLISHED 15 Feb 2022