Europe: COVID-19 Vaccines – Announcements/Regulatory Actions/Deployment
European Medicines Agency
News & Press Releases
News: Meeting highlights from the Committee for Medicinal Products for Human Use (CHMP) 21-24 February 2022 (new)
CHMP, Last updated: 25/02/2022
News: EMA recommends authorisation of booster doses of Comirnaty from 12 years of age (new)
CHMP, Last updated: 24/02/2022
News: EMA recommends approval of Spikevax for children aged 6 to 11 (new)
CHMP, PDCO, Last updated: 24/02/2022
::::::
European Centre for Disease Prevention and Control
https://www.ecdc.europa.eu/en
Latest Updates [Selected]
No new digest content identified.
::::::
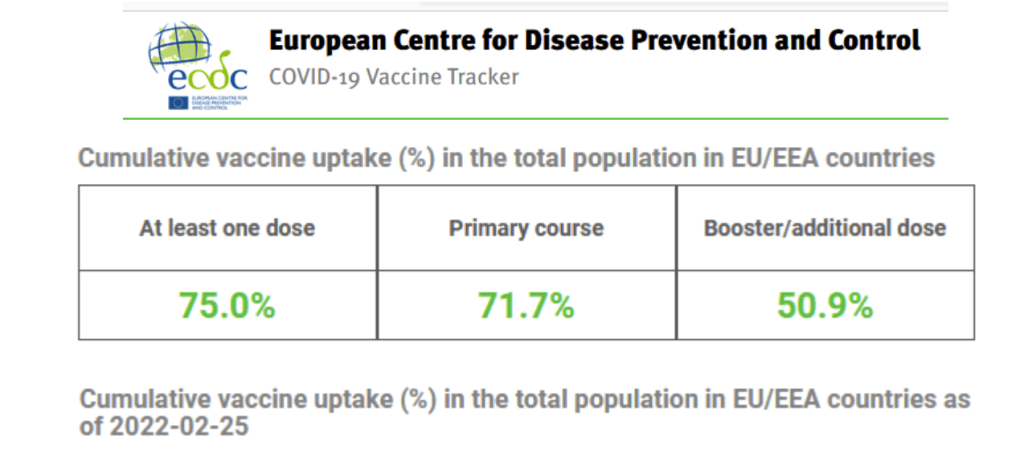
Accessed 26 Feb 2022
https://vaccinetracker.ecdc.europa.eu/public/extensions/COVID-19/vaccine-tracker.html#uptake-tab
::::::
European Commission
https://ec.europa.eu/commission/presscorner/home/en
Statement 25 February 2022
World Rare Disease Day: Statement by Commissioner Stella Kyriakides
Today, more than 6,000 rare diseases are affecting up to 36 million people in the EU. For those living with rare diseases – many of them children – quality of life is strongly affected, as they often cause chronic pain and suffering, and some can be life-threatening.