Lancet Global Health
Mar 2022 Volume 10 Number 3 e298-e447
https://www.thelancet.com/journals/langlo/issue/current
Bridging the gap between science and policy in global health governance
Olivier Nay, Françoise Barré-Sinoussi
Lancet Global Health
Mar 2022 Volume 10 Number 3 e298-e447
https://www.thelancet.com/journals/langlo/issue/current
Bridging the gap between science and policy in global health governance
Olivier Nay, Françoise Barré-Sinoussi
Lancet Infectious Diseases
Mar 2022 Volume 22 Number 3 p297-426, e66-e100
https://www.thelancet.com/journals/laninf/issue/current
Editorial
Transitioning to endemicity with COVID-19 research
The Lancet Infectious Diseases
Lancet Infectious Diseases
Mar 2022 Volume 22 Number 3 p297-426, e66-e100
https://www.thelancet.com/journals/laninf/issue/current
Comment
COVID-19 vaccine inequity, dependency, and production capability in low-income and middle-income countries: the case of Bangladesh
Abdullah Mahmud-Al-Rafat, et al.
Lancet Infectious Diseases
Mar 2022 Volume 22 Number 3 p297-426, e66-e100
https://www.thelancet.com/journals/laninf/issue/current
Articles
Efficacy and safety of the CVnCoV SARS-CoV-2 mRNA vaccine candidate in ten countries in Europe and Latin America (HERALD): a randomised, observer-blinded, placebo-controlled, phase 2b/3 trial
Peter G Kremsner, et al. on behalf of the HERALD Study Group
Lancet Infectious Diseases
Mar 2022 Volume 22 Number 3 p297-426, e66-e100
https://www.thelancet.com/journals/laninf/issue/current
Effectiveness of an inactivated virus-based SARS-CoV-2 vaccine, BBV152, in India: a test-negative, case-control study
Devashish Desai, et al
Lancet Infectious Diseases
Mar 2022 Volume 22 Number 3 p297-426, e66-e100
https://www.thelancet.com/journals/laninf/issue/current
Lancet Infectious Diseases
Mar 2022 Volume 22 Number 3 p297-426, e66-e100
https://www.thelancet.com/journals/laninf/issue/current
Safety and efficacy of a three-dose regimen of Plasmodium falciparum sporozoite vaccine in adults during an intense malaria transmission season in Mali: a randomised, controlled phase 1 trial
Mahamadou S Sissoko, et al
Lancet Infectious Diseases
Mar 2022 Volume 22 Number 3 p297-426, e66-e100
https://www.thelancet.com/journals/laninf/issue/current
Efficacy, immunogenicity, and safety of a quadrivalent HPV vaccine in men: results of an open-label, long-term extension of a randomised, placebo-controlled, phase 3 trial
Stephen E Goldstone, et al
Lancet Respiratory Medicine
Mar 2022 Volume 10 Number 3 p221-312, e25-e33
https://www.thelancet.com/journals/lanres/issue/current
Editorial
Future pandemics: failing to prepare means preparing to fail
The Lancet Respiratory Medicine
Nature
Volume 603 Issue 7899, 3 March 2022
https://www.nature.com/nature/volumes/603/issues/7899
Editorial | 28 February 2022
Wanted: better systems for turning evidence into action
The pandemic created a colossal demand for scientific evidence to inform decision-making. Now researchers are mapping out what went wrong and what needs to change.
Nature Reviews Genetics
Volume 23 Issue 3, March 2022
https://www.nature.com/nrg/volumes/23/issues/3
Review Article | 26 November 2021
Navigating the pitfalls of applying machine learning in genomics
Machine learning is widely applied in various fields of genomics and systems biology. In this Review, the authors describe how responsible application of machine learning requires an understanding of several common pitfalls that users should be aware of (and mitigate) to avoid unreliable results.
Sean Whalen, Jacob Schreiber, Katherine S. Pollard
Nature Reviews Immunology
Volume 22 Issue 3, March 2022
https://www.nature.com/nri/volumes/22/issues/3
Comment | 10 February 2022
Modelling vaccination strategies for COVID-19
Mathematical models have been used to guide strategies for COVID-19 vaccine distribution. But with the emergence of new SARS-CoV-2 variants and waning immunity, how should vaccine distribution be prioritized?
Caroline E. Wagner, Chadi M. Saad-Roy, Bryan T. Grenfell
New England Journal of Medicine
March 3, 2022 Vol. 386 No. 9
https://www.nejm.org/toc/nejm/medical-journal
Final Analysis of Efficacy and Safety of Single-Dose Ad26.COV2.S J. Sadoff and Others
New England Journal of Medicine
March 3, 2022 Vol. 386 No. 9
https://www.nejm.org/toc/nejm/medical-journal
njp Vaccines
https://www.nature.com/npjvaccines/
[Accessed 05 Mar 2022]
Article
02 Mar 2022
Pandemic-response adenoviral vector and RNA vaccine manufacturing
Zoltán Kis, Kyungjae Tak, Cleo Kontoravdi
Pediatrics
Volume 149, Issue 3, March 1, 2022
https://pediatrics.aappublications.org/
Articles
Household Transmission and Clinical Features of SARS-CoV-2 Infections
Huong Q. McLean, PhD, MPH; Carlos G. Grijalva, MD, MPH; Kayla E. Hanson, MPH; Yuwei Zhu, MD, MS; Jessica E. Deyoe, MPH …
PharmacoEconomics
Volume 40, issue 3, March 2022
https://link.springer.com/journal/40273/volumes-and-issues/40-3
Original Research Article
Public Preferences for a COVID-19 Vaccination Program in Quebec: A Discrete Choice Experiment
Authors – Gabin F. Morillon, Thomas G. Poder
Open Access
Published: 20 January 2022
Pages: 341 – 354
PLoS Medicine
http://www.plosmedicine.org/
(Accessed 05 Mar 2022)
Ethnic inequalities in COVID-19 vaccine uptake and comparison to seasonal influenza vaccine uptake in Greater Manchester, UK: A cohort study
Ruth Elizabeth Watkinson, Richard Williams, Stephanie Gillibrand, Caroline Sanders, Matt Sutton
Research Article | published 03 Mar 2022 PLOS Medicine
https://doi.org/10.1371/journal.pmed.1003932
PLoS One
http://www.plosone.org/
[Accessed 05 Mar 2022]
Research Article
Willingness to take COVID-19 vaccination in low-income countries: Evidence from Ethiopia
Christoph Strupat, Zemzem Shigute, Arjun S. Bedi, Matthias Rieger
Research Article | published 03 Mar 2022 PLOS ONE
https://doi.org/10.1371/journal.pone.0264633
PLoS One
http://www.plosone.org/
[Accessed 05 Mar 2022]
Implementation of a COVID-19 Genomic Surveillance Regional Network for Latin America and Caribbean region
Juliana Almeida Leite, Andrea Vicari, Enrique Perez, Marilda Siqueira, Paola Resende, Fernando Couto Motta, Lucas Freitas, Jorge Fernandez, Barbara Parra, Andrés Castillo, Rodrigo Fasce, Alexander Augusto Martinez Caballero, COVID-19 Genomic Surveillance Regional Network , Lionel Gresh, Sylvain Aldighieri, Jean-Marc Gabastou, Leticia Franco, Jairo Mendez-Rico
Research Article | published 03 Mar 2022 PLOS ONE
https://doi.org/10.1371/journal.pone.0252526
PLoS One
http://www.plosone.org/
[Accessed 05 Mar 2022]
Public opinion concerning governments’ response to the COVID-19 pandemic
Cathy W. S. Chen, Tsai-Hung Fan
Research Article | published 02 Mar 2022 PLOS ONE
https://doi.org/10.1371/journal.pone.0260062
PLoS One
http://www.plosone.org/
[Accessed 05 Mar 2022]
Vaccination conspiracy beliefs among social science & humanities and STEM educated people—An analysis of the mediation paths
Željko Pavić, Adrijana Šuljok
Research Article | published 01 Mar 2022 PLOS ONE
https://doi.org/10.1371/journal.pone.0264722
PLoS One
http://www.plosone.org/
[Accessed 05 Mar 2022]
COVID-19 vaccine acceptance and rejection in an adult population in Bosnia and Herzegovina
Adnan Fojnica, Ahmed Osmanovic, Nermin Đuzic, Armin Fejzic, Ensar Mekic, Zehra Gromilic, Imer Muhovic, Amina Kurtovic-Kozaric
Research Article | published 28 Feb 2022 PLOS ONE
https://doi.org/10.1371/journal.pone.0264754
PLoS One
http://www.plosone.org/
[Accessed 05 Mar 2022]
COVID-19 vaccination coverage in deprived populations living in segregated colonies: A nationwide cross-sectional study in Hungary
János Sándor, Ferenc Vincze, Maya Liza Shrikant, László Kőrösi, László Ulicska, Karolina Kósa, Róza Ádány
Research Article | published 28 Feb 2022 PLOS ONE
https://doi.org/10.1371/journal.pone.0264363
PNAS – Proceedings of the National Academy of Sciences of the United States
February 8, 2022 | vol. 119 | no. 6
https://www.pnas.org/toc/pnas/119/6
Research Article February 1, 2022Open Access
A 680,000-person megastudy of nudges to encourage vaccination in pharmacies
Encouraging vaccination is a pressing policy problem. To assess whether text-based reminders can encourage pharmacy vaccination and what kinds of messages work best, we conducted a megastudy. We randomly assigned 689,693 Walmart pharmacy patients to …
Katherine L. Milkman, Linnea Gandhi, […] Angela L. Duckworth
Public Health Reports
Volume 137 Issue 2, March/April 2022
https://journals.sagepub.com/toc/phrg/137/2
Brief Report
Surveillance of COVID-19 Vaccination in Nursing Homes, United States, December 2020–July 2021
Andrew I. Geller, MD, Daniel S. Budnitz, MD, MPH, Heather Dubendris, MSPH, Radhika Gharpure, DVM, MPH, Minn Soe, MBBS, MPH, Hsiu Wu, MD, Elizabeth J. Kalayil, MPH, Andrea L. Benin, MD, Suchta A. Patel, DO, MPH, Megan C. Lindley, MPH, Ruth Link-Gelles, PhD, MPH
First Published February 5, 2022; pp. 239–243
Public Health Reports
Volume 137 Issue 2, March/April 2022
https://journals.sagepub.com/toc/phrg/137/2
Ethical Principles in Personal Protective Equipment Inventory Management Decisions and Partnerships Across State Lines During the COVID-19 Pandemic
Tazim Merchant, BA, Sean Hormozian, BS, Roger S. Smith, BS, Tricia Pendergrast, BA, Aliza Siddiqui, BS, Zhaoyang Wen, BA, Mark Sheldon, PhD
First Published December 30, 2021; pp. 208–212
Public Health Reports
Volume 137 Issue 2, March/April 2022
https://journals.sagepub.com/toc/phrg/137/2
Commentary
Ensuring Equitable COVID-19 Vaccination for People With Disabilities and Their Caregivers
Lisa D. Wiggins, PhD, Harriet Jett, JD, MPA, Jennifer Meunier, MPH
First Published December 30, 2021; pp. 185–189
Qualitative Health Research
Volume 32 Issue 4, March 2022
https://journals.sagepub.com/toc/qhra/current
Research Articles
How Perceptions of Responsibility and Affective Consequences Influence Parents’ Digital Media Engagement in Relation to Human Papillomavirus Vaccination
Maja Nordtug
First Published December 29, 2021; pp. 683–693
Research Ethics
Volume 18 Issue 1, January 2022
http://journals.sagepub.com/toc/reab/current
Topic Piece
Reshaping the review of consent so we might improve participant choice Hugh Davieson behalf of the Oxford “A” Research Ethics Committee
First Published September 15, 2021; pp. 3–12
Research Ethics
Volume 18 Issue 1, January 2022
http://journals.sagepub.com/toc/reab/current
Original Article: Empirical
Recruiting pupils for a school-based eye study in Nigeria: Trust and informed consent concerns Ferdinand Chinedum Maduka-Okafor, Onochie Ike Okoye, Ngozi Oguego, Nnenma Udeh, Ada Aghaji, Obiekwe Okoye, Ifeoma R Ezegwui, Emmanuel Amaechi Nwobi, Euzebus Ezugwu, Ernest Onwasigwe, Rich E Umeh, Chiamaka Aneji
First Published September 8, 2021; pp. 13–23
Risk Management and Healthcare Policy
https://www.dovepress.com/risk-management-and-healthcare-policy-archive56
[Accessed 05 Mar 2022]
Original Research
Understanding the Facilitators and Barriers to COVID-19 Vaccine Uptake Among Teachers in the Sagnarigu Municipality of Northern Ghana: A Cross-Sectional Study
Dubik SD
Published Date: 24 February 2022
Science
Volume 375| Issue 6584| 4 Mar 2022
https://www.science.org/toc/science/current
Policy Forum
A role for funders in fostering China’s research integrity
Li Tang
03 Mar 2022: 979-981
Free
Despite recent progress, challenges remain
Science Translational Medicine
Volume 14| Issue 634| 2 Mar 2022
https://www.science.org/toc/stm/current
Report
Antibodies elicited by SARS-CoV-2 infection or mRNA vaccines have reduced neutralizing activity against Beta and Omicron pseudoviruses
BY Benjamin L. Sievers et al.
Vaccine
Volume 40, Issue 7 Pages 967-1060 (11 February 2022)
https://www.sciencedirect.com/journal/vaccine/vol/40/issue/7
Short communication Open access
Adolescents’ attitudes to the COVID-19 vaccination
W.H.S. Wong, D. Leung, G.T. Chua, J.S.R. Duque, … Y.L. Lau
Pages 967-969
Vaccine
Volume 40, Issue 7 Pages 967-1060 (11 February 2022)
https://www.sciencedirect.com/journal/vaccine/vol/40/issue/7
Short communication Open access
Research articleAbstract only
Cost-effectiveness of pertussis booster vaccination for preschool children in Japan
Motoko Tanaka, Reiko Okubo, Shu-Ling Hoshi, Nobuyuki Ishikawa, Masahide Kondo
Pages 1010-1018
Vaccine
Volume 40, Issue 7 Pages 967-1060 (11 February 2022)
https://www.sciencedirect.com/journal/vaccine/vol/40/issue/7
Research article Open access
Variation in influenza vaccine assessment, receipt, and refusal by the concentration of Medicare Advantage enrollees in U.S. nursing homes
Patience Moyo, Elliott Bosco, Barbara H. Bardenheier, Maricruz Rivera-Hernandez, … Andrew R. Zullo
Pages 1031-1037
Value in Health
March 2022 Volume 25 Issue 3 p321-472
https://www.valueinhealthjournal.com/current
THEMED SECTION: ARTIFICIAL INTELLIGENCE
The Value of Artificial Intelligence for Healthcare Decision Making—Lessons Learned
Danielle Whicher, Thomas Rapp
Published online: January 31, 2022
p328-330
Value in Health
March 2022 Volume 25 Issue 3 p321-472
https://www.valueinhealthjournal.com/current
BRIEF REPORT
The Use of Cost-Effectiveness Thresholds for Evaluating Health Interventions in Low- and Middle-Income Countries From 2015 to 2020: A Review
Joseph Kazibwe, Adrian Gheorghe, David Wilson, Francis Ruiz, Kalipso Chalkidou, Y-Ling Chi
Published online: October 28, 2021
p385-389
Gates Open Research
https://gatesopenresearch.org/browse/articles
[Accessed 05 Mar 2022]
[No new digest content identified]
medRxiv
https://www.medrxiv.org/content/about-medrxiv
medRxiv is a free online archive and distribution server for complete but unpublished manuscripts (preprints) in the medical, clinical, and related health sciences. Preprints are preliminary reports of work that have not been certified by peer review. They should not be relied on to guide clinical practice or health-related behavior and should not be reported in news media as established information. medRxiv is for the distribution of preprints – complete but unpublished manuscripts – that describe human health research conducted, analyzed, and interpreted according to scientific principles…
Precision recruitment for high-risk participants in a COVID-19 research study
Aziz Mezlini, Eamon Caddigan, Allison Shapiro, Ernesto Ramirez, Helena Kondow-McConaghy, Justin Yang, Kerry DeMarco, Pejman Naraghi-Arani, Luca Foschini
medRxiv 2022.03.03.22271504; doi: https://doi.org/10.1101/2022.03.03.22271504
Impact of Population Mixing Between a Vaccinated Majority and Unvaccinated Minority on Disease Dynamics. Implications for SARS-CoV-2
Ashleigh Tuite, Afia Amoako, David Fisman
medRxiv 2021.12.14.21267742; doi: https://doi.org/10.1101/2021.12.14.21267742
Determinants of COVID-19 vaccine acceptability in Mozambique: the role of institutional trust
Bo Hu, Wei Yang, Paul Alexandre Bouanchaud, Yolanda Chongo, Jennifer Wheeler, Sergio Chicumbe, Marcos Chissano
medRxiv 2022.03.03.22271828; doi: https://doi.org/10.1101/2022.03.03.22271828
Abstract
Background Vaccination plays an imperative role in protecting public health and preventing avoidable mortality. Yet, the reasons for vaccine hesitancy are not well understood. This study investigates the factors associated with the acceptability of COVID-19 vaccine in Mozambique. Methods The data came from the three waves of the COVID-19 Knowledge, Attitudes and Practices (KAP) survey which followed a cohort of 1,371 adults in Mozambique over three months (N=3,809). Data collection was through a structured questionnaire using telephone interviewing (CAPI). Multilevel regression analysis was conducted to identify the trajectories of, and the factors associated with COVID-19 vaccine acceptability. Results There was great volatility in COVID-19 vaccine acceptability over time. Institutional trust was consistently and strongly correlated with different measures of vaccine acceptability. There was a greater decline in vaccine acceptability in people with lower institutional trust. The positive correlation between institutional trust and vaccine acceptability was stronger in younger than older adults. Vaccine acceptability also varied by gender and marital status. Conclusions Vaccine acceptability is sensitive to news and information circulated in the public domain. Institutional trust is a central driver of vaccine acceptability and contributes to the resilience of the health system. Our study highlights the importance of health communication and building a trustful relationship between the general public and public institutions in the context of a global pandemic.
Acute and long-term impacts of COVID-19 on economic vulnerability: a population-based longitudinal study (COVIDENCE UK)
Anne E Williamson, Florence Tydeman, Alec Miners, Kate Pyper, Adrian R Martineau
medRxiv 2022.03.03.22271835; doi: https://doi.org/10.1101/2022.03.03.22271835
An observational study of the association between COVID-19 vaccination rates and participation in a vaccine lottery
Dajung Jun, Anthony Scott
medRxiv 2022.03.02.22271734; doi: https://doi.org/10.1101/2022.03.02.22271734
Abstract
Objectives Are financial incentives from entry in a vaccine lottery associated with a higher probability of vaccination for COVID-19? Design A cross-sectional study with adjustment for covariates using logistic regression Setting October and November 2021, Australia. Participants 2,375 respondents of the Taking the Pulse of the Nation Survey Interventions Participation in the Million Dollar Vaccination Lottery Primary and secondary outcome measures The proportion of respondents who had any vaccination, a first dose only, or second dose compared to all other respondents Results Those who participated in the lottery were 2.27 times more likely to be vaccinated after the lottery opened on October 1st than those who did not. This was driven by those receiving second doses. Lottery participants were 1.38 times more likely to receive their first dose after October 1st and 2.31 times more likely to receive their second dose after October 1st. Conclusions Lottery participation is associated with a higher vaccination rate, with this effect dominated by a higher rate of second doses. There is a smaller insignificant difference for those receiving a first dose, suggesting lotteries may not be as effective at reducing vaccine hesitancy, compared to ‘nudging’ people to get their second dose more quickly.
Prevalence of Chronic Diseases, Depression, and Stress among U.S. Child Care Professionals during the COVID-19 Pandemic
Jad A. Elharake, Mehr Shafiq, Ayse Cobanoglu, Amyn A. Malik, Madeline Klotz, John Eric Humphries, Thomas Murray, Kavin M. Patel, David Wilkinson, Inci Yildirim, Rachel Diaz, Rosalia Rojas, Anael Kuperwajs Cohen, Aiden Lee, Chin R. Reyes, Saad B. Omer, Walter S. Gilliam
medRxiv 2022.03.01.22271717; doi: https://doi.org/10.1101/2022.03.01.22271717
The Serological Sciences Network (SeroNet) for COVID-19: Depth and Breadth of Serology Assays and Plans for Assay Harmonization
Amy B. Karger, James D. Brien, Jayne M. Christen, Santosh Dhakal, Troy J. Kemp, Sabra L. Klein, Ligia A. Pinto, Lakshmanane Premkumar, John D. Roback, Raquel A. Binder, Karl W. Boehme, Suresh Boppana, James M. Crawford, John L. Daiss, Alan P. Dupuis II, Ana M. Espino, Catherine Forconi, J. Craig Forrest, Roxie C. Girardin, Douglas A. Granger, Steve W. Granger, Natalie S. Haddad, Christopher D. Heaney, Danielle T. Hunt, Joshua L. Kennedy, Christopher L. King, Kate Kruczynski, Joshua LaBaer, F. Eun-Hyung Lee, William T. Lee, Shan-Lu Liu, Gerard Lozanski, Todd Lucas, Ann M. Moormann, Vel Murugan, Nkemakonam C. Okoye, Petraleigh Pantoja, Anne F. Payne, Jin Park, Swetha Pinninti, Amelia K. Pinto, Nora Pisanic, Ji Qiu, Carlos A. Sariol, Lusheng Song, Tara L. Steffen, E. Taylor Stone, Linda M. Styer, Mehul S. Suthar, Stefani N. Thomas, Bharat Thyagarajan, Jennifer L. Yates, Kimia Sobhani
medRxiv 2022.02.27.22271399; doi: https://doi.org/10.1101/2022.02.27.22271399
Racial/Ethnic Disparities in the Observed COVID-19 Case Fatality Rate Among the U.S. Population
L. Philip Schumm, Mihai C. Giurcanu, Kenneth J. Locey, Jean Czerlinski Ortega, Zhenyu Zhang, Robert L. Grossman
medRxiv 2022.03.01.22271708; doi: https://doi.org/10.1101/2022.03.01.22271708
Healthcare services access, use, and barriers among migrants in Europe: a systematic review
Petros Galanis, Koureas Spyros, Olga Siskou, Olympia Konstantakopoulou, Georgios Angelopoulos, Daphne Kaitelidou
medRxiv 2022.02.24.22271449; doi: https://doi.org/10.1101/2022.02.24.22271449
Assessing the Burden of COVID-19 in Developing Countries: Systematic Review, Meta-Analysis, and Public Policy Implications
Andrew Levin, Nana Owusu-Boaitey, Sierra Pugh, Bailey K. Fosdick, Anthony B. Zwi, Anup Malani, Satej Soman, Lonni Besançon, Ilya Kashnitsky, Sachin Ganesh, Aloysius McLaughlin, Gayeong Song, Rine Uhm, Daniel Herrera-Esposito, Gustavo de los Campos, Ana Carolina Pecanha Antiono, Enyew Birru Tadese, Gideon Meyerowitz-Katz
medRxiv 2021.09.29.21264325; doi: https://doi.org/10.1101/2021.09.29.21264325
Vaccine hesitancy strongly correlates with COVID-19 deaths underreporting
Adam Sobieszek, Miriam Lipniacka, Tomasz Lipniacki
medRxiv 2022.02.27.22271579; doi: https://doi.org/10.1101/2022.02.27.22271579
Genomic surveillance of SARS-CoV-2 in a university community: insights into tracking variants, transmission, and spread of Gamma (P.1) variant
Ilinca I. Ciubotariu, Jack Dorman, Nicole M. Perry, Lev Gorenstein, Jobin J. Kattoor, Abebe A. Fola, Amy Zine, G. Kenitra Hendrix, Rebecca P. Wilkes, Andrew Kitchen, Giovanna Carpi
medRxiv 2022.02.25.22271521; doi: https://doi.org/10.1101/2022.02.25.22271521
SARS-CoV-2 mRNA Vaccination-Associated Myocarditis in Children Ages 12-17: A Stratified National Database Analysis
Tracy Beth Høeg, Allison Krug, Josh Stevenson, John Mandrola
medRxiv 2021.08.30.21262866; doi: https://doi.org/10.1101/2021.08.30.21262866
Wellcome Open Research [to 05 Mar 2022]
https://wellcomeopenresearch.org/browse/articles
[Accessed 05 Mar 2022]
Wellcome Open Research provides all Wellcome researchers with a place to rapidly publish any results they think are worth sharing. All articles benefit from rapid publication, transparent peer review and editorial guidance on making all source data openly available.
Method Article metrics
Revised
An optimization of four SARS-CoV-2 qRT-PCR assays in a Kenyan laboratory to support the national COVID-19 rapid response teams [version 2; peer review: 1 approved, 1 approved with reservations]
Khadija Said Mohammed, Zaydah R. de Laurent, Donwilliams O. Omuoyo, Clement Lewa, Elijah Gicheru, Robinson Cheruiyot, Brian Bartilol, Shadrack Mutua, Jennifer Musyoki, Horace Gumba, Jedidah Mwacharo, Debra Riako, Shaban J. Mwangi, Bonface M. Gichuki, Lydia Nyamako, Angela Karani, Henry Karanja, Daisy Mugo, John N. Gitonga, Susan Njuguna, Wilson Gumbi, Brian Tawa, Metrine Tendwa, Wesley Cheruiyot, Yiakon Sein, John K. Nyambu, Shem O. Patta, Thani Suleiman Thani, Eric K. Maitha, Benson Kitole, Mohamed S. Mwakinangu, Barke S. Muslih, John Ochieng Otieno, Joyce U. Nyiro, Patience Kiyuka, Leonard Ndwiga, Kevin Wamae, Domtila Kimani, Johnstone Makale, John Mwita Morobe, Victor Osoti, Arnold W. Lambisia, Calleb Odundo, Salim Mwarumba, Martin Mutunga, Philip Bejon, Benjamin Tsofa, Charles N. Agoti, Lynette Isabella Ochola-Oyier
Peer Reviewers Davis Nwakanma; Esther Omuseni and John Waitumbi
Funders
Wellcome Trust
DELTAS Africa Initiative
PATH
National Institute for Health Research
Department for International Development, UK Government
LATEST VERSION PUBLISHED 04 Mar 2022
Research Article metrics AWAITING PEER REVIEW
Pandemic preparedness and responsiveness of research review committees: lessons from review of COVID-19 protocols at KEMRI Wellcome Trust Research Programme in Kenya [version 1; peer review: awaiting peer review]
Alex Hinga, Lisha Jeena, Esther Awuor, Jane Kahindi, Marianne Munene, Samson Kinyanjui, Sassy Molyneux, Vicki Marsh, Dorcas Kamuya
Peer Reviewers Invited
Funders
Wellcome Trust
African Academy of Sciences
PUBLISHED 03 Mar 2022
Research Article metrics AWAITING PEER REVIEW
Health care users’ acceptance of broad consent for storage of biological materials and associated data for research purposes in Uganda [version 1; peer review: awaiting peer review]
Hellen Nansumba, Mugalula Flaviano, Semanda Patrick, Ssewanyana Isaac, Douglas Wassenaar
Peer Reviewers Invited
Funder
Wellcome Trust
PUBLISHED 01 Mar 2022
Pre-Print Servers
Gates Open Research
https://gatesopenresearch.org/browse/articles
[Accessed 05 Mar 2022]
[No new digest content identified]
medRxiv
https://www.medrxiv.org/content/about-medrxiv
medRxiv is a free online archive and distribution server for complete but unpublished manuscripts (preprints) in the medical, clinical, and related health sciences. Preprints are preliminary reports of work that have not been certified by peer review. They should not be relied on to guide clinical practice or health-related behavior and should not be reported in news media as established information. medRxiv is for the distribution of preprints – complete but unpublished manuscripts – that describe human health research conducted, analyzed, and interpreted according to scientific principles…
Precision recruitment for high-risk participants in a COVID-19 research study
Aziz Mezlini, Eamon Caddigan, Allison Shapiro, Ernesto Ramirez, Helena Kondow-McConaghy, Justin Yang, Kerry DeMarco, Pejman Naraghi-Arani, Luca Foschini
medRxiv 2022.03.03.22271504; doi: https://doi.org/10.1101/2022.03.03.22271504
Impact of Population Mixing Between a Vaccinated Majority and Unvaccinated Minority on Disease Dynamics. Implications for SARS-CoV-2
Ashleigh Tuite, Afia Amoako, David Fisman
medRxiv 2021.12.14.21267742; doi: https://doi.org/10.1101/2021.12.14.21267742
Determinants of COVID-19 vaccine acceptability in Mozambique: the role of institutional trust
Bo Hu, Wei Yang, Paul Alexandre Bouanchaud, Yolanda Chongo, Jennifer Wheeler, Sergio Chicumbe, Marcos Chissano
medRxiv 2022.03.03.22271828; doi: https://doi.org/10.1101/2022.03.03.22271828
Abstract
Background Vaccination plays an imperative role in protecting public health and preventing avoidable mortality. Yet, the reasons for vaccine hesitancy are not well understood. This study investigates the factors associated with the acceptability of COVID-19 vaccine in Mozambique. Methods The data came from the three waves of the COVID-19 Knowledge, Attitudes and Practices (KAP) survey which followed a cohort of 1,371 adults in Mozambique over three months (N=3,809). Data collection was through a structured questionnaire using telephone interviewing (CAPI). Multilevel regression analysis was conducted to identify the trajectories of, and the factors associated with COVID-19 vaccine acceptability. Results There was great volatility in COVID-19 vaccine acceptability over time. Institutional trust was consistently and strongly correlated with different measures of vaccine acceptability. There was a greater decline in vaccine acceptability in people with lower institutional trust. The positive correlation between institutional trust and vaccine acceptability was stronger in younger than older adults. Vaccine acceptability also varied by gender and marital status. Conclusions Vaccine acceptability is sensitive to news and information circulated in the public domain. Institutional trust is a central driver of vaccine acceptability and contributes to the resilience of the health system. Our study highlights the importance of health communication and building a trustful relationship between the general public and public institutions in the context of a global pandemic.
Acute and long-term impacts of COVID-19 on economic vulnerability: a population-based longitudinal study (COVIDENCE UK)
Anne E Williamson, Florence Tydeman, Alec Miners, Kate Pyper, Adrian R Martineau
medRxiv 2022.03.03.22271835; doi: https://doi.org/10.1101/2022.03.03.22271835
An observational study of the association between COVID-19 vaccination rates and participation in a vaccine lottery
Dajung Jun, Anthony Scott
medRxiv 2022.03.02.22271734; doi: https://doi.org/10.1101/2022.03.02.22271734
Abstract
Objectives Are financial incentives from entry in a vaccine lottery associated with a higher probability of vaccination for COVID-19? Design A cross-sectional study with adjustment for covariates using logistic regression Setting October and November 2021, Australia. Participants 2,375 respondents of the Taking the Pulse of the Nation Survey Interventions Participation in the Million Dollar Vaccination Lottery Primary and secondary outcome measures The proportion of respondents who had any vaccination, a first dose only, or second dose compared to all other respondents Results Those who participated in the lottery were 2.27 times more likely to be vaccinated after the lottery opened on October 1st than those who did not. This was driven by those receiving second doses. Lottery participants were 1.38 times more likely to receive their first dose after October 1st and 2.31 times more likely to receive their second dose after October 1st. Conclusions Lottery participation is associated with a higher vaccination rate, with this effect dominated by a higher rate of second doses. There is a smaller insignificant difference for those receiving a first dose, suggesting lotteries may not be as effective at reducing vaccine hesitancy, compared to ‘nudging’ people to get their second dose more quickly.
Prevalence of Chronic Diseases, Depression, and Stress among U.S. Child Care Professionals during the COVID-19 Pandemic
Jad A. Elharake, Mehr Shafiq, Ayse Cobanoglu, Amyn A. Malik, Madeline Klotz, John Eric Humphries, Thomas Murray, Kavin M. Patel, David Wilkinson, Inci Yildirim, Rachel Diaz, Rosalia Rojas, Anael Kuperwajs Cohen, Aiden Lee, Chin R. Reyes, Saad B. Omer, Walter S. Gilliam
medRxiv 2022.03.01.22271717; doi: https://doi.org/10.1101/2022.03.01.22271717
The Serological Sciences Network (SeroNet) for COVID-19: Depth and Breadth of Serology Assays and Plans for Assay Harmonization
Amy B. Karger, James D. Brien, Jayne M. Christen, Santosh Dhakal, Troy J. Kemp, Sabra L. Klein, Ligia A. Pinto, Lakshmanane Premkumar, John D. Roback, Raquel A. Binder, Karl W. Boehme, Suresh Boppana, James M. Crawford, John L. Daiss, Alan P. Dupuis II, Ana M. Espino, Catherine Forconi, J. Craig Forrest, Roxie C. Girardin, Douglas A. Granger, Steve W. Granger, Natalie S. Haddad, Christopher D. Heaney, Danielle T. Hunt, Joshua L. Kennedy, Christopher L. King, Kate Kruczynski, Joshua LaBaer, F. Eun-Hyung Lee, William T. Lee, Shan-Lu Liu, Gerard Lozanski, Todd Lucas, Ann M. Moormann, Vel Murugan, Nkemakonam C. Okoye, Petraleigh Pantoja, Anne F. Payne, Jin Park, Swetha Pinninti, Amelia K. Pinto, Nora Pisanic, Ji Qiu, Carlos A. Sariol, Lusheng Song, Tara L. Steffen, E. Taylor Stone, Linda M. Styer, Mehul S. Suthar, Stefani N. Thomas, Bharat Thyagarajan, Jennifer L. Yates, Kimia Sobhani
medRxiv 2022.02.27.22271399; doi: https://doi.org/10.1101/2022.02.27.22271399
Racial/Ethnic Disparities in the Observed COVID-19 Case Fatality Rate Among the U.S. Population
L. Philip Schumm, Mihai C. Giurcanu, Kenneth J. Locey, Jean Czerlinski Ortega, Zhenyu Zhang, Robert L. Grossman
medRxiv 2022.03.01.22271708; doi: https://doi.org/10.1101/2022.03.01.22271708
Healthcare services access, use, and barriers among migrants in Europe: a systematic review
Petros Galanis, Koureas Spyros, Olga Siskou, Olympia Konstantakopoulou, Georgios Angelopoulos, Daphne Kaitelidou
medRxiv 2022.02.24.22271449; doi: https://doi.org/10.1101/2022.02.24.22271449
Assessing the Burden of COVID-19 in Developing Countries: Systematic Review, Meta-Analysis, and Public Policy Implications
Andrew Levin, Nana Owusu-Boaitey, Sierra Pugh, Bailey K. Fosdick, Anthony B. Zwi, Anup Malani, Satej Soman, Lonni Besançon, Ilya Kashnitsky, Sachin Ganesh, Aloysius McLaughlin, Gayeong Song, Rine Uhm, Daniel Herrera-Esposito, Gustavo de los Campos, Ana Carolina Pecanha Antiono, Enyew Birru Tadese, Gideon Meyerowitz-Katz
medRxiv 2021.09.29.21264325; doi: https://doi.org/10.1101/2021.09.29.21264325
Vaccine hesitancy strongly correlates with COVID-19 deaths underreporting
Adam Sobieszek, Miriam Lipniacka, Tomasz Lipniacki
medRxiv 2022.02.27.22271579; doi: https://doi.org/10.1101/2022.02.27.22271579
Genomic surveillance of SARS-CoV-2 in a university community: insights into tracking variants, transmission, and spread of Gamma (P.1) variant
Ilinca I. Ciubotariu, Jack Dorman, Nicole M. Perry, Lev Gorenstein, Jobin J. Kattoor, Abebe A. Fola, Amy Zine, G. Kenitra Hendrix, Rebecca P. Wilkes, Andrew Kitchen, Giovanna Carpi
medRxiv 2022.02.25.22271521; doi: https://doi.org/10.1101/2022.02.25.22271521
SARS-CoV-2 mRNA Vaccination-Associated Myocarditis in Children Ages 12-17: A Stratified National Database Analysis
Tracy Beth Høeg, Allison Krug, Josh Stevenson, John Mandrola
medRxiv 2021.08.30.21262866; doi: https://doi.org/10.1101/2021.08.30.21262866
Wellcome Open Research [to 05 Mar 2022]
https://wellcomeopenresearch.org/browse/articles
[Accessed 05 Mar 2022]
Wellcome Open Research provides all Wellcome researchers with a place to rapidly publish any results they think are worth sharing. All articles benefit from rapid publication, transparent peer review and editorial guidance on making all source data openly available.
Method Article metrics
Revised
An optimization of four SARS-CoV-2 qRT-PCR assays in a Kenyan laboratory to support the national COVID-19 rapid response teams [version 2; peer review: 1 approved, 1 approved with reservations]
Khadija Said Mohammed, Zaydah R. de Laurent, Donwilliams O. Omuoyo, Clement Lewa, Elijah Gicheru, Robinson Cheruiyot, Brian Bartilol, Shadrack Mutua, Jennifer Musyoki, Horace Gumba, Jedidah Mwacharo, Debra Riako, Shaban J. Mwangi, Bonface M. Gichuki, Lydia Nyamako, Angela Karani, Henry Karanja, Daisy Mugo, John N. Gitonga, Susan Njuguna, Wilson Gumbi, Brian Tawa, Metrine Tendwa, Wesley Cheruiyot, Yiakon Sein, John K. Nyambu, Shem O. Patta, Thani Suleiman Thani, Eric K. Maitha, Benson Kitole, Mohamed S. Mwakinangu, Barke S. Muslih, John Ochieng Otieno, Joyce U. Nyiro, Patience Kiyuka, Leonard Ndwiga, Kevin Wamae, Domtila Kimani, Johnstone Makale, John Mwita Morobe, Victor Osoti, Arnold W. Lambisia, Calleb Odundo, Salim Mwarumba, Martin Mutunga, Philip Bejon, Benjamin Tsofa, Charles N. Agoti, Lynette Isabella Ochola-Oyier
Peer Reviewers Davis Nwakanma; Esther Omuseni and John Waitumbi
Funders
Wellcome Trust
DELTAS Africa Initiative
PATH
National Institute for Health Research
Department for International Development, UK Government
LATEST VERSION PUBLISHED 04 Mar 2022
Research Article metrics AWAITING PEER REVIEW
Pandemic preparedness and responsiveness of research review committees: lessons from review of COVID-19 protocols at KEMRI Wellcome Trust Research Programme in Kenya [version 1; peer review: awaiting peer review]
Alex Hinga, Lisha Jeena, Esther Awuor, Jane Kahindi, Marianne Munene, Samson Kinyanjui, Sassy Molyneux, Vicki Marsh, Dorcas Kamuya
Peer Reviewers Invited
Funders
Wellcome Trust
African Academy of Sciences
PUBLISHED 03 Mar 2022
Research Article metrics AWAITING PEER REVIEW
Health care users’ acceptance of broad consent for storage of biological materials and associated data for research purposes in Uganda [version 1; peer review: awaiting peer review]
Hellen Nansumba, Mugalula Flaviano, Semanda Patrick, Ssewanyana Isaac, Douglas Wassenaar
Peer Reviewers Invited
Funder
Wellcome Trust
PUBLISHED 01 Mar 2022
Think Tanks
Brookings
http://www.brookings.edu/
Accessed 05 Mar 2022
Africa in Focus
How strengthened political engagement can lead to improved health outcomes in Africa
Jean-Baptiste Nikiema
Tuesday, March 1, 2022
Center for Global Development [to 05 Mar 2022]
https://www.cgdev.org/
Accessed 05 Mar 2022
Expanding Emergency Vaccine Manufacturing Capacity in Latin America and the Caribbean
Publication
March 3, 2022
The COVID-19 pandemic has transformed the global vaccine manufacturing landscape, with capacity more than doubling in the past 18 months. Before the pandemic, manufacturers produced an estimated total of five billion vaccine doses each year. In 2021, manufacturers produced 12 billion doses of COVID-19 vaccine, with 1.4 billion doses produced in December alone.
How Can Latin America and the Caribbean Successfully Expand Emergency Vaccine Manufacturing?
March 3, 2022
The COVID-19 pandemic has pushed global vaccine manufacturing capacity to new limits and primed the pump for further expansion in regions like Lati
Morgan Pincombe and Javier Guzman
Chatham House [to 05 Mar 2022]
https://www.chathamhouse.org/
Accessed 05 Mar 2022
[No new digest content identified]
CSIS
https://www.csis.org/
Accessed 05 Mar 2022
Upcoming Event
Overcoming Gender-Related Barriers to Immunization Services
March 8, 2022
Podcast Episode
Live From Munich: Dr. Seth Berkley: “It is a Security Issue”
March 4, 2022 | By Katherine E. Bliss, J. Stephen Morrison
Kaiser Family Foundation
https://www.kff.org/search/?post_type=press-release
Accessed 05 Mar 2022
March 1, 2022 News Release
Large Shares of the Public Worry about the Consequences of Both Ending and Keeping COVID-19 Restrictions, with Partisans Largely Split on Which Direction is Most Concerning
As federal, state, and local authorities move to roll back COVID-19 restrictions, a new KFF COVID-19 Vaccine Monitor survey finds many people ready to get back to normal but a public also nervous about the potential consequences. Large shares of the public are worried about the implications of both keeping…
Rand [to 05 Mar 2022]
https://www.rand.org/pubs.html
Reports, Selected Journal Articles
[No new digest content identified]
Vaccines and Global Health: The Week in Review is a weekly digest summarizing news, events, announcements, peer-reviewed articles and research in the global vaccine ethics and policy space. Content is aggregated from key governmental, NGO, international organization and industry sources, key peer-reviewed journals, and other media channels. This summary proceeds from the broad base of themes and issues monitored by the Center for Vaccine Ethics & Policy in its work: it is not intended to be exhaustive in its coverage. You are viewing the blog version of our weekly digest, typically comprised of between 30 and 40 posts below all dated with the current issue date
.– Request an Email Summary: Vaccines and Global Health : The Week in Review is published as a single email summary, scheduled for release each Saturday evening before midnight (EDT in the U.S.). If you would like to receive the email version, please send your request to david.r.curry@centerforvaccineethicsandpolicy.org.
– pdf version: A pdf of the current issue is available here:
– blog edition: comprised of the approx. 35+ entries posted below.
– Twitter: Readers can also follow developments on twitter: @vaxethicspolicy.
.
– Links: We endeavor to test each link as we incorporate it into any post, but recognize that some links may become “stale” as publications and websites reorganize content over time. We apologize in advance for any links that may not be operative. We believe the contextual information in a given post should allow retrieval, but please contact us as above for assistance if necessary.
Support this knowledge-sharing service: Your financial support helps us cover our costs and to address a current shortfall in our annual operating budget. Click here to donate and thank you in advance for your contribution.
.
David R. Curry, MS
Executive Director
Center for Vaccine Ethics and Policy
::::::
Remembering Dr. Paul Farmer
World-renowned infectious disease doctor, anthropologist, global health advocate, and author leaves large legacy
Posted on Feb 21, 2022

Partners In Health announced that its founder, Dr. Paul Farmer, unexpectedly passed away today in Rwanda from an acute cardiac event while he was sleeping. Dr. Farmer was 62 years old. He is survived by his wife, Didi Bertrand Farmer, and their three children.
Partners In Health CEO Dr. Sheila Davis released the following statement: “Paul Farmer’s loss is devastating, but his vision for the world will live on through Partners in Health. Paul taught all those around him the power of accompaniment, love for one another, and solidarity. Our deepest sympathies are with his family.”
::::::
About Dr. Paul Farmer
Paul Farmer, M.D., Ph.D., was Kolokotrones University Professor and chair of the Department of Global Health and Social Medicine at Harvard Medical School, chief of the Division of Global Health Equity at Brigham and Women’s Hospital in Boston, and co-founder and chief strategist of Partners In Health.
Dr. Farmer and his colleagues pioneered novel, community-based treatment strategies that demonstrate the delivery of high-quality health care in resource-poor settings. He wrote extensively on health, human rights, and the consequences of social inequality. Dr. Farmer was a member of the American Academy of Arts and Sciences and the Institute of Medicine of the National Academy of Sciences, from which he was the recipient of the 2018 Public Welfare Medal.
He authored multiple books, including: In the Company of the Poor: Conversations with Dr. Paul Farmer and Fr. Gustavo Gutiérrez, Reimagining Global Health: An Introduction, and To Repair the World: Paul Farmer Speaks to the Next Generation. His most recent book was released in November 2020: Fevers, Feuds, and Diamonds: Ebola and the Ravages of History.
Inequity ‘a Moral Indictment of Our Times’, Secretary-General Tells Universal Vaccination Debate, Saying It Gives COVID Free Reign to Circulate Unchecked
Secretary-General Statements and Messages
25 February 2022 SG/SM/21159
Following is the text of Secretary-General António Guterres’ video remarks to the high-level thematic debate on “Galvanizing Momentum for Universal Vaccination”, in New York today:
Mr. President of the General Assembly, Excellencies, ladies and gentlemen,
Let me begin by thanking the President of the General Assembly for convening this critical meeting focused on galvanizing momentum for universal vaccination.
We have the tools and the know-how to end the COVID-19 pandemic this year. But we have a long way to go. We are nowhere near meeting the WHO [World Health Organization] goal to vaccinate 70 per cent of people in all countries by the middle of this year.
Yes, over 10 billion doses of COVID-19 vaccines have been administered globally. But this number masks the stark inequity in vaccine access. High-income countries have administered 13 times more doses per person than low-income countries. Eighty-five per cent of the people of Africa have not received a single vaccine dose.
This inequity is a moral indictment of our times. It costs lives. It damages economies. And it gives the virus free reign to circulate unchecked and mutate, eroding hard-won gains and threatening the whole world.
Ending the pandemic requires ensuring access to tests, vaccines and treatments for everyone, everywhere. In recent months, deliveries of COVID-19 vaccines have been steadily increasing. This month marked COVAX’s delivery of 1 billion doses to 92 low- and middle-income countries.
But much more is needed. Galvanizing momentum means countries fulfilling and accelerating vaccine dose-sharing and donation commitments to COVAX with better quality of supply. It means manufacturers prioritizing and fulfilling vaccine contracts with COVAX, ensuring full transparency on monthly production and creating the conditions for the local or regional production of tests, vaccines and treatments.
This includes pharmaceutical companies more rapidly sharing licences, know-how and technology. Regional production is critical for sustainable supply. It means significant investments in fragile health and economic infrastructure. It means donors and international financial institutions stepping up with the necessary support. And it means fighting the plague of vaccine misinformation.
We have seen hopeful progress when supply is secured and predictable … when doses are donated with ample shelf-life … and when there is a deep understanding of what a country needs to accelerate vaccinations.
Let’s build on that momentum together. In the coming days, weeks and months let us use every opportunity — through the G20, the World Health Assembly, the G7 and the General Assembly — to mobilize ambitious action to meet the WHO vaccination strategy targets and ensure no one is left behind. If we do it right, we won’t just end this pandemic, we will begin a truly meaningful effort to prevent future ones and build a safer, healthier world for all.
Thank you.
WHO Director-General as Guest Lecture at Robert S. McNamara Lecture on War and Peace, Harvard Kennedy School – 25 February 2022
25 February 2022
Speech [Excerpt]
…The authors of WHO’s Constitution were well aware of the link between health and peace, which is why they wrote in the preamble that the health of all peoples is fundamental to the attainment of peace and security, and is dependent upon the fullest co-operation of individuals and States.
Since those words were written, the world has faced many outbreaks and epidemics. Just this century, we have seen H5N1 influenza, SARS, MERS, the H1N1 pandemic, multiple Ebola outbreaks, Zika and more.
But of course, nothing matches the scale of the COVID-19 pandemic, which has thrown the world into turmoil for more than two years. COVID-19 is a powerful demonstration that a pandemic is so much more than a health crisis. It illustrates the interconnectedness between health and the economy, security, education, and the intimate links between the health of humans, animals and our planet. There are many lessons to learn about what has worked and what has not. Let me suggest five:
The first is that science must guide policy, not the other way round.
Throughout the pandemic, WHO has convened thousands of scientists from around the world to examine the rapidly emerging evidence and distil it into the guidance we give the world.
Just this week, we have convened a research and innovation forum to identify the most pressing research priorities and chart the way forward.
Science has given us valuable insights into how this virus spreads, how it causes disease, and how to stop it. But in some countries and communities, and on social media, the marginalization and politicization of science has impeded the response to the pandemic and cost lives. Politics undermining science. My point is not that science should be the only consideration in decision-making about public health. My point is that science should be the central and guiding consideration.
The second lesson is that science can in fact widen inequalities, unless it is paired with a commitment to equity.
I’m sure that most or all of you are vaccinated. And yet as we speak, 83% of the population of Africa is yet to receive a single dose of vaccine. Vaccine nationalism, export bans and bilateral deals between manufacturers and high-income nations severely restricted the number of doses COVAX was able to ship in the first half of last year. The supply situation has now improved, and COVAX has been able to ship more than 1.2 billion doses of vaccine to 144 countries and territories.
WHO and our partners are working night and day to support countries to turn vaccines into vaccinations, to reach our target of vaccinating 70% of the population of every country by the middle of this year. To reach that target, we are calling on all countries to urgently fill the ACT Accelerator’s financing gap of US$16 billion, to ensure equitable access to vaccines, tests and treatments and PPE everywhere.
The third lesson is that a resilient health system is not the same thing as an advanced medical care system.
Even some countries with the most sophisticated medical care were overwhelmed by COVID-19.
By contrast, some middle-income countries with fewer resources fared much better, thanks to investments in public health after outbreaks of SARS, MERS, H1N1 and others, especially in the Mekong region.
For instance, the simple art of contact tracing is one that many high-income countries have struggled with, but it’s one that many low- and middle-income countries have done well, because of their experience with infectious disease outbreaks, and their investments in public health. The backbone of public health is robust primary health care, for detecting outbreaks at the earliest possible stage, as well as for preventing disease and promoting health at the community level.
The fourth lesson is that the world needs a new agreement that sets the rules of the game for responding to epidemics and pandemics.
Instead of a coherent and cohesive global response, the pandemic has been marked by a chaotic patchwork of responses, which in some cases have punished countries for doing the right thing, as in the case of the travel bans imposed on South Africa and Botswana when they first reported the emergence of the Omicron variant.
And the fifth lesson is that trust is everything.
A study published in The Lancet earlier this month examined the reasons why some countries have had higher rates of infection and death than others from COVID-19. The age profile of the country, GDP per capita, and mean body mass index were all found to play a part. But the researchers found that perhaps the single most important factor in countries’ preparedness and ability to respond effectively is trust.
The study concluded that stronger risk communication and community engagement are essential for making the world safer against future epidemics and pandemics.
Vaccines, diagnostics, therapeutics and other tools are essential, but the most effective tool is engaged and empowered communities…
IFPMA – Three priorities to urgently increase access to COVID-19 vaccines
25 February 2022
[Excerpts]
…Today, over 12 billion doses of vaccines have been produced, and more than 60 percent of the world’s population have received at least one dose.[1] Getting as many people as possible vaccinated remains critical to our pandemic response. Even in the face of new variants, including most recently Omicron, current vaccines show continued protection from infection and significant effectiveness against hospitalization and death. For those people who do contract COVID-19 or are at-risk, several approved treatments are now an important and integral part of combatting COVID-19.
Whilst significant progress has been made, COVID-19 vaccines are still not reaching equitably all priority populations worldwide. In May 2021, …biopharmaceutical companies publicly committed to continue working with governments, international institutions, and non-governmental organizations to address vaccine inequity. Over the past six months, governments that have significant domestic supplies of COVID-19 vaccines have increasingly shared them with low- and lower-middle-income countries and vaccine manufacturers have continued to further ramp up production, including through voluntary licensing and technology transfer across several continents. By the end of January 2022, COVAX delivered its first billion vaccine doses.[2] In addition, over 3 billion doses have been delivered to low- and lower-middle-income countries. Moving forwards, we expect that a significant number of doses will continue to be delivered to low- and lower-middle-income countries.
Our collective efforts are materializing, but to achieve an improvement in vaccine equity, manufacturers, governments, international institutions, and other non-governmental organizations must redouble efforts to support countries as they mobilize to execute national vaccine rollouts and remove barriers to the efficient distribution and administration of vaccine doses, so that they reach those who need them most.
To support this endeavor,…biopharmaceutical companies will continue to work with all relevant stakeholders on the following three overarching priorities and supporting activities:
STEP UP SUPPORT FOR COUNTRY READINESS TO ROLL OUT COVID-19 VACCINE DOSES:
Work collaboratively with governments and established procurement mechanisms, such as COVAX and the African Vaccine Acquisition Trust (AVAT), to improve COVID-19 vaccine demand forecasting, alignment of deliveries and donations, distribution and administration, particularly in low- and lower-middle income countries;
Continue engaging and sharing information with COVAX and other relevant organizations to ensure countries have better visibility of COVAX deliveries and donations;
Provide timely information to assist with regulatory system streamlining and appropriate flexibilities to smooth the path for the reallocation of doses, and enable swift approval of new COVID-19 vaccines and manufacturing facilities;
Continue to increase confidence in vaccines and vaccination programs, particularly by countering misinformation, providing timely vaccine effectiveness and safety data, and supporting adequate no-fault compensation programs;
Continue to encourage governments to expand the number of vaccinators in countries to be able to rapidly deploy supply.
CONTRIBUTE TO EQUITABLE DISTRIBUTION OF COVID-19 VACCINE DOSES:
Continue to work with governments that have significant supplies of COVID-19 vaccines to improve dose sharing with low- and lower-middle-income countries in a responsible and timely way, through COVAX or other efficient established mechanisms such as AVAT;
Expend every effort to make uncommitted COVID-19 vaccine doses available with urgency to prioritized populations in low- and lower-middle income countries through COVAX or other efficient established mechanisms;
Work with relevant authorities to facilitate recognition of an extension to the COVID-19 vaccine shelf-life and batch-level extensions in line with ongoing studies looking at the stability of vaccines, to avoid vaccine wastage;
Encourage the elimination of remaining trade and regulatory barriers to support cross-border supply and free flow of goods, raw materials, services, and personnel needed for COVID-19 vaccine manufacturing, distribution, and administration;
Support efforts that tackle supply chain and logistic bottlenecks to increase vaccination rates.
CONTINUE TO DRIVE INNOVATION:
Continue to prioritize research to develop the next generation of COVID-19 vaccines that can provide long lasting and strong protection against variants of concern, and address logistical issues in storage, delivery, and administration;
Urge governments to guarantee unhindered access to pathogens (samples and sequences) of any COVID-19 variants to support the rapid development of potential new vaccines and treatments;
Continue to optimize production of vaccines, without compromising safety and quality, including through existing and additional voluntary collaborations with partners that can produce significant quantities.
WHO and NAM [National Academy of Medicine] encourage digital platforms to apply global principles for identifying credible sources of health information
24 February 2022
The World Health Organization (WHO) and the National Academy of Medicine (NAM) are encouraging social media companies and other digital platforms to apply global principles for identifying credible sources of health information in their channels. The principles, originally published in a 2021 NAM paper, were evaluated by an international group of public health experts convened by WHO and BMJ in December 2021. The WHO meeting report is now available for download.
“Digital platforms have a uniquely powerful opportunity to enable worldwide access to high-quality health information,” said NAM president Victor J. Dzau. “We hope these principles can serve as a launchpad for productive collaboration among industry, public health, and consumer representatives to protect and improve the health of people everywhere.”
“The rapid spread of health misinformation through digital platforms has become a serious threat to public health globally,” said Andy Pattison, Team Lead, Digital Channels, Department of Digital Health and Innovation, WHO. “Since the COVID-19 pandemic began, WHO has worked with technology companies to keep people safe and informed by improving accessibility of high-quality health information online. It is every platform’s responsibility to protect the safety and health of their users. For this reason, we encourage all digital platforms to incorporate the new global principles for identifying credible sources of health information in their guidelines, safety policies and enforcement to protect public health.”
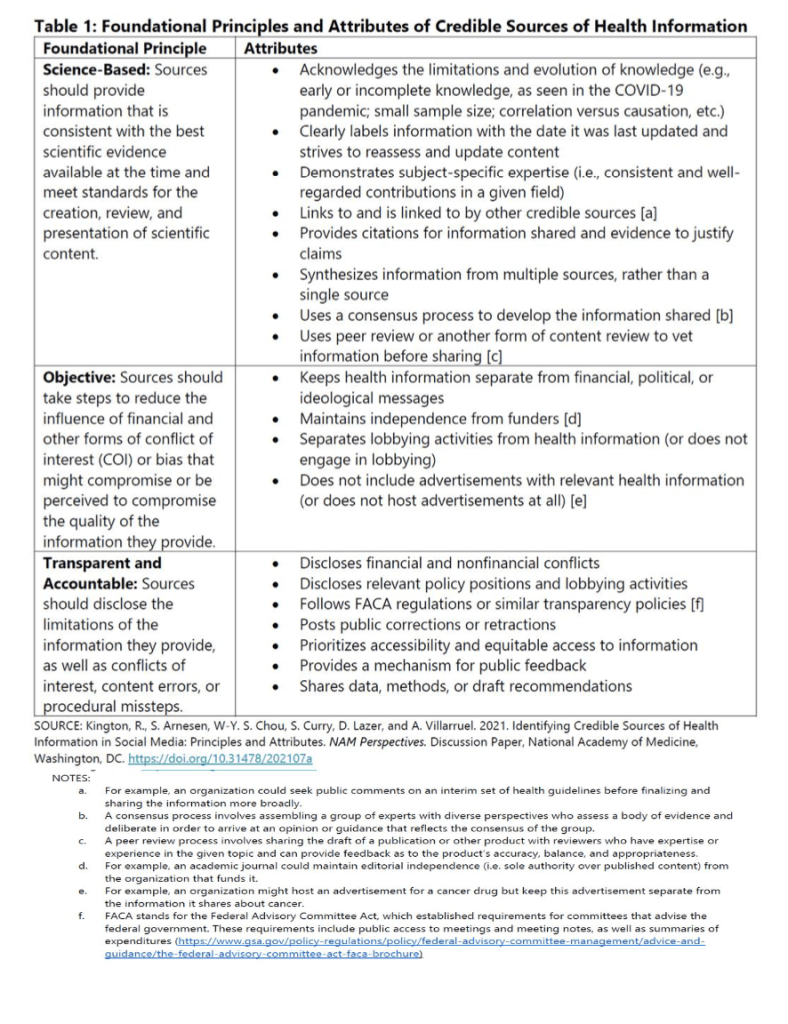
The principles state that, to be considered credible, sources should be science-based, objective, transparent, and accountable. The NAM paper provides a list of material attributes that platforms and consumers can use to assess a source’s alignment with these principles.
Participants at the December 2021 WHO meeting agreed that the principles and attributes are generally applicable to global contexts. However, they identified potential implementation challenges, noting that “factors such as culture, language, the influence of the source of information and political views within the country can affect what is seen as credible.”…
World Economic Forum [to 26 Feb 2022]
https://agenda.weforum.org/news/
Media [Selected]
Public Thinks COVID-19 Is Here to Stay, Global Survey Finds
News 24 Feb 2022
Most adults surveyed across 30 countries agree that even if maximum measures were to be maintained COVID-19 will never be fully eradicated
However, support for mandatory vaccination or proof of vaccination in certain public locations varies wildly across different regions and countries
Generally, Asian and Latin American countries are the most supportive of maintaining vaccine mandates, while Eastern and Central Europe and the United States were the most opposed
Read more about the World Economic Forum-Ipsos Global Survey on COVID-19 Expectations and Vaccination here
A new Ipsos survey for the World Economic Forum finds that, on average across 30 countries, 71% of adults do not expect that COVID-19 will ever stop spreading entirely. A majority of adults in every country – from 51% in China to 85% in the Netherlands – agree that “even with all the measures being taken, we will never be able to fully stop the spread of COVID-19 and variants.”
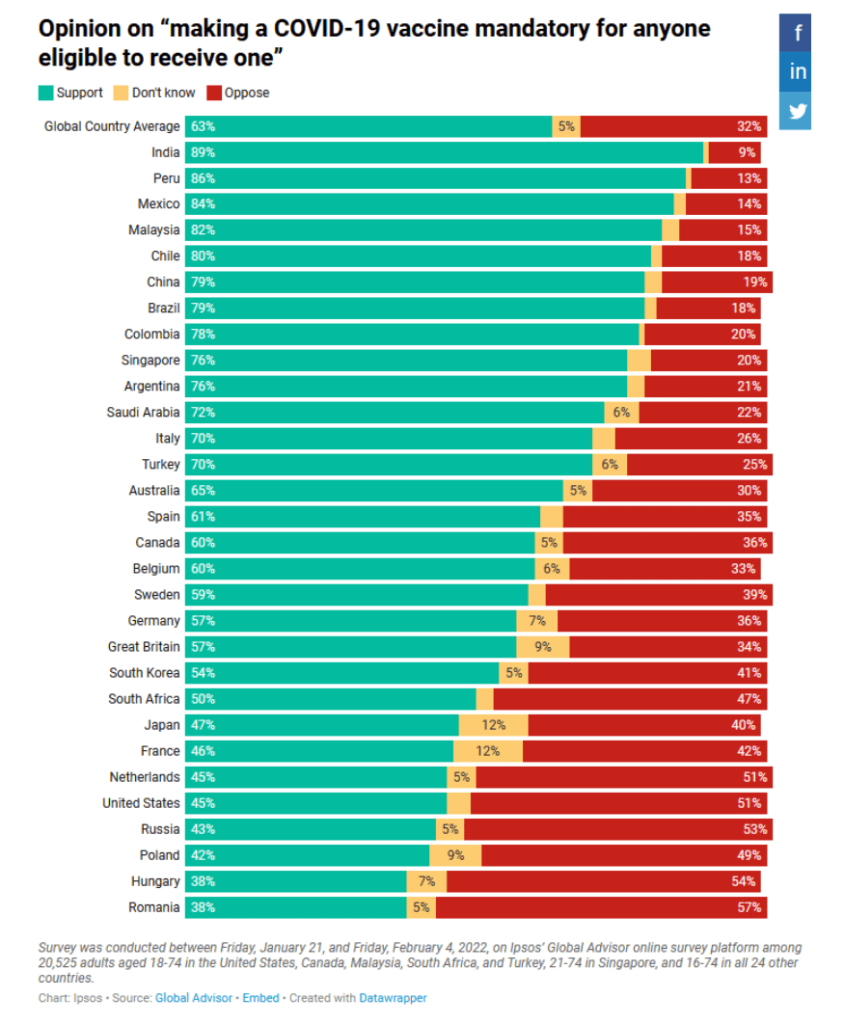
However, the survey reveals vastly different levels of popular support for mandatory vaccination across countries. In India, China, Malaysia, Singapore, and throughout Latin America, more than 75% support “making a COVID-19 vaccine mandatory for anyone eligible to receive one.” In most of Central and Eastern Europe as well as in the United States, majorities are opposed to it. Views about requiring proof of vaccination to be allowed in various facilities (e.g., sporting venues, restaurants, workplaces) mirror those on mandatory vaccination and vary similarly across countries.
Over the past year, support for vaccination mandates has grown significantly in China, Italy, Australia, Germany, and France, but it has decreased in Japan and the U.S.
These are the main findings of a survey of 20,525 adults aged 18-74 from 30 countries conducted by Ipsos on its Global Advisor online platform between January 21 and February 4, 2022…
…Support for mandatory COVID-19 vaccination
On average across all 30 countries surveyed, 63% support “making a COVID-19 vaccine mandatory for anyone eligible for it” while 32% oppose it and 5% don’t know. Support exceeds 75% in emerging countries of Asia and Latin America, led by India (89%) Peru (86%). In contrast, opposition dominates in Romania (57%), Hungary (54%), Russia (53%), the United States (51%), and the Netherlands (51%).
::::::
Coronavirus [COVID-19] – WHO
Public Health Emergency of International Concern (PHEIC)
https://www.who.int/emergencies/diseases/novel-coronavirus-2019
Weekly Epidemiological and Operational updates
Last update: 25 Feb 2022
Confirmed cases :: 430 257 564
Confirmed deaths :: 5 922 049
Vaccine doses administered: 10 407 359 583
::::::
Weekly epidemiological update on COVID-19 – 22 February 2022
Overview
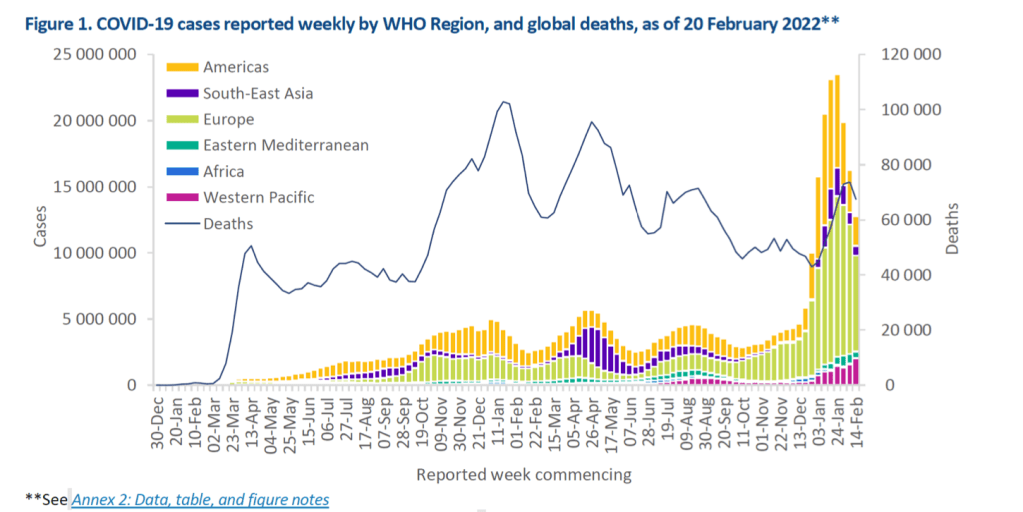
Globally, during the week of 14 to 20 February 2022, the number of new COVID-19 cases and deaths decreased by 21% and 8% respectively, compared to the previous week. Across the six WHO regions, over 12 million new cases and over 67 000 new deaths were reported. As of 20 February 2022, over 422 million confirmed cases and over 5.8 million deaths have been reported globally.
At the regional level, the Western Pacific Region reported a 29% increase in the number of new weekly cases while all other regions reported decreases. The number of new weekly deaths increased in the Western Pacific (+21%) and the African (+20%) regions, and decreased in the South-East Asia (-37%), the Regions of the Americas (-9%), the European (-5%) and Eastern Mediterranean regions (-4%).
In this edition we provide an update on:
The geographic distribution of circulating SARS-CoV-2 variants of concern (VOCs), including the prevalence and summary of current evidence of the Omicron variant. We also provide updates on vaccine effectiveness for the Delta and Omicron variants.
::::::
Statement on Omicron sublineage BA.2
WHO 22 February 2022
As part of its on-going work to track variants, WHO’s Technical Advisory Group on SARS-CoV-2 Virus Evolution (TAG-VE) met yesterday to discuss the latest evidence on the Omicron variant of concern, including its sublineages BA.1 and BA.2.
Based on available data of transmission, severity, reinfection, diagnostics, therapeutics and impacts of vaccines, the group reinforced that the BA.2 sublineage should continue to be considered a variant of concern and that it should remain classified as Omicron. The group emphasized that BA.2 should continue to be monitored as a distinct sublineage of Omicron by public health authorities.
The Omicron variant of concern is currently the dominant variant circulating globally, accounting for nearly all sequences reported to GISAID. Omicron is made up of several sublineages, each of them being monitored by WHO and partners. Of them, the most common ones are BA.1, BA.1.1 (or Nextstrain clade 21K) and BA.2 (or Nextstrain clade 21L). At a global level, the proportion of reported sequences designated BA.2 has been increasing relative to BA.1 in recent weeks, however the global circulation of all variants is reportedly declining.
BA.2 differs from BA.1 in its genetic sequence, including some amino acid differences in the spike protein and other proteins. Studies have shown that BA.2 has a growth advantage over BA.1. Studies are ongoing to understand the reasons for this growth advantage, but initial data suggest that BA.2 appears inherently more transmissible than BA.1, which currently remains the most common Omicron sublineage reported. This difference in transmissibility appears to be much smaller than, for example, the difference between BA.1 and Delta. Further, although BA.2 sequences are increasing in proportion relative to other Omicron sublineages (BA.1 and BA.1.1), there is still a reported decline in overall cases globally…
::::::
WHO Director General Speeches [selected]
https://www.who.int/director-general/speeches
Selected
25 February 2022
Speech
WHO Director-General as Guest Lecture at Robert S. McNamara Lecture on War and Peace, Harvard Kennedy School – 25 February 2022
[See Perspectives above for excerpt]
25 February 2022
Speech
WHO Director-General’s opening remarks at High-Level Thematic Debate: Galvanizing Momentum for Universal Vaccination, President of the 76th Session of the General Assembly – 25 February 2022
24 February 2022
Speech
WHO Director-General’s opening remarks at COVID-19 Global research & innovation forum – 24 February 2022
24 February 2022
Speech
WHO Director-General’s opening remarks at first meeting of the Intergovernmental Negotiating Body to draft and negotiate a WHO convention, agreement or other international instrument on pandemic prevention, preparedness and response – 24 February 2022
[Excerpt]
…This is a momentous undertaking, and a necessary one. Because the COVID-19 pandemic has shown that the status quo is not good enough to protect our communities, our societies, and our economies.
The pandemic has exposed and exacerbated fundamental weaknesses in pandemic preparedness and response at both the national and the global levels:
:: Complex and fragmented governance and lack of leadership;
:: Inadequate financing;
:: And insufficient systems and tools.
Instead of solidarity, the pandemic has been marred by inequity.
Equity does not mean providing access to tools when there is a surplus, when the privileged have used what they need. It is about timely access, so that all people have access to all the available tools, at the same time.
The lack of trust or the trust deficit between and within countries, partners and stakeholders has shattered our unified defence during the pandemic. Voluntary mechanisms have not solved and will not solve these challenges.
Global health security is too important to be left to chance, or goodwill, or shifting geopolitical currents, or the vested interests of companies and shareholders. Recognizing that our fates as a global community are intertwined, the World Health Assembly established this intergovernmental negotiating body during a special session, which, by the way, is only the second time it had held such an extraordinary meeting.
Today’s meeting is the start of an historic opportunity for Member States to work together to strengthen the world’s structures and systems for pandemic preparedness and response.
While we are operating under an ambitious timeline, we need to take the lessons learned from the COVID-19 pandemic and use them to build back better. The aim should be a world better prepared to prevent pandemic threats and respond to them when they do occur, in at least five ways:
First, by building national, regional and global capacities for preparing and responding to pandemics and other global health emergencies, based on a whole-of-government and whole-of-society approach;
Second, by establishing global access and benefit sharing for all pathogens, and determining a global policy for the equitable production and distribution of countermeasures;
Third, by establishing robust systems and tools for pandemic preparedness and response;
Fourth, by building a long-term plan for sustainable financing, so that support for global health threat management and response systems is shared by all;
And fifth, by empowering WHO to fulfil its mandate as the directing and coordinating authority on international health work, including for pandemic preparedness and response
At the Special Session, the World Health Assembly specifically noted the importance of broad engagement to ensure a successful outcome. So we encourage all Member States to participate in this process and support the work of the INB, in developing a new international instrument on pandemic prevention, preparedness and response.
Now begins the critical process of coming together around a common goal – health – for the future of our children and their children.
Together, let us chart a way forward, for this and future generations, to better prevent, prepare and respond to future pandemics and health emergencies.
23 February 2022
Speech
WHO Director-General’s remarks at the Preparatory Meeting of the Global Fund’s Seventh Replenishment – 23 February 2022
23 February 2022
Speech
WHO Director-General’s opening remarks at the media briefing on COVID-19 – 23 February 2022
22 February 2022
Speech
WHO Director-General’s remarks at ILO Multisectoral Policy Forum: “Building a human-centered recovery from the COVID-19 crisis” – 22 February 2022
Status of COVID-19 Vaccines within WHO EUL/PQ evaluation process 23 December 2021
[Full scale view available at title link above]
[Updated on 18 February 2022]
COVID Vaccines/Therapeutics – Developer/Manufacturer Announcements
[Selected press releases/announcements from organizations from WHO EUL/PQ listing above and other organizations]
AstraZeneca
Press Releases – No new digest announcements identified
Bharat Biotech
Press Releases – No new digest announcements identified
BioCubaFarma – Cuba
Últimas Noticias – Website not leading at inquiry
Biontech
Press Releases
Pfizer and BioNTech Receive Positive CHMP Opinion for COVID-19 Vaccine Booster in Adolescents 12 through 17 Years of Age in the European Union
24 February 202
CanSinoBIO
News – [Website not responding at inquiry]
Clover Biopharmaceuticals – China
News – No new digest announcements identified
Curevac [Bayer Ag – Germany]
News – Website not responding at inquiry
Gamaleya National Center
Latest News and Events – See Russia below
IMBCAMS, China
Home – Website not responding at inquiry
Janssen/JNJ
Press Releases – No new digest announcements identified
Moderna
Press Releases
February 24, 2022
EMA Committee for Medicinal Products for Human Use (CHMP) Adopts Positive Opinion Recommending Authorization for The Use of The Moderna Covid-19 Vaccine in Children (6-11 Years) In the European Union
February 23, 2022
Moderna and Thermo Fisher Scientific Announce Long-Term Strategic Collaboration
Agreement to leverage dedicated commercial fill-finish manufacturing capacity in the US for mRNA vaccines and therapies
Novavax
Press Releases
Novavax Announces Shipments of its COVID-19 Vaccine to European Union Member States
Feb 23, 2022
Pfizer
Recent Press Releases
02.24.2022
Pfizer and BioNTech Receive Positive CHMP Opinion for COVID-19 Vaccine Booster in Adolescents 12 through 17 Years of Age in the European Union
Sanofi Pasteur
Press Releases
February 23 2022
Press releases
Sanofi and GSK to seek regulatory authorization for COVID-19 vaccine
* Final analysis of the global VAT02 booster trial confirms universal ability to boost neutralizing antibodies 18- to 30-fold across vaccine platforms (mRNA, adenovirus)
* In the VAT08 Phase 3 primary series trial, two doses of the Sanofi-GSK vaccine in seronegative populations demonstrated:
* 100% efficacy against severe COVID-19 disease and hospitalizations
* 75% efficacy against moderate or severe COVID-19 disease
* 57.9% efficacy against any symptomatic COVID-19 disease, in line with expected vaccine effectiveness in today’s environment dominated by variants of concern
* Favorable safety profile following both primary series and booster vaccinations
Serum Institute of India
NEWS & ANNOUNCEMENTS – No new digest announcements identified
Shifa Pharmed [Iran]
http://shafapharmed.com/
No news page identified.
Sinopharm/WIBPBIBP
News – No new digest announcements identified
Sinovac
Press Releases – No new digest announcements identified
Vector State Research Centre of Viralogy and Biotechnology
Home – No new digest announcements identified
Zhifei Longcom, China
[Anhui Zhifei Longcom Biologic Pharmacy Co., Ltd.]
[No website identified]
::::::
GSK
Press releases for media
Medicago and GSK announce the approval by Health Canada of COVIFENZ®, an adjuvanted plant-based COVID-19 vaccine
24 February 2022 Medicago, a biopharmaceutical company headquartered in Quebec City, and GlaxoSmithKline (GSK) today announced that Health Canada has granted approval for COVIFENZ®, COVID-19 vaccine, (plant-based virus-like particles [VLP], recombinant, adjuvanted). This vaccine is indicated for active immunization to prevent coronavirus disease 2019 (COVID19) caused by severe acute respiratory syndrome coronavirus 2 (SARSCoV2) in individuals 18 to 64 years of age…
23 February 2022 Sanofi and GSK to seek regulatory authorization for COVID-19 vaccine
Merck
News releases – No new digest announcements identified
Novartis
News – No new digest announcements identified
SK Biosciences
Press releases – No new digest announcements identified
Valneva
Press Releases
February 25, 2022
Valneva Receives Initial CHMP Assessment of its Inactivated, Adjuvanted COVID-19 Vaccine Candidate VLA2001
February 21, 2022
Valneva Awarded Up to £20 Million by Scottish Enterprise to Advance Vaccine Development