The Lancet
Apr 16, 2022 Volume 399 Number 10334 p1441-1572, e37
https://www.thelancet.com/journals/lancet/issue/current
Comment
Understanding of COVID-19 from infection–fatality ratio
Xiaoying Gu, Bin Cao
The Lancet
Apr 16, 2022 Volume 399 Number 10334 p1441-1572, e37
https://www.thelancet.com/journals/lancet/issue/current
Comment
Understanding of COVID-19 from infection–fatality ratio
Xiaoying Gu, Bin Cao
The Lancet
Apr 16, 2022 Volume 399 Number 10334 p1441-1572, e37
https://www.thelancet.com/journals/lancet/issue/current
Understanding of COVID-19 from infection–fatality ratio
Xiaoying Gu, Bin Cao
The Lancet
Apr 16, 2022 Volume 399 Number 10334 p1441-1572, e37
https://www.thelancet.com/journals/lancet/issue/current
Articles
Variation in the COVID-19 infection–fatality ratio by age, time, and geography during the pre-vaccine era: a systematic analysis
COVID-19 Forecasting Team
Open Access
The Lancet
Apr 16, 2022 Volume 399 Number 10334 p1441-1572, e37
https://www.thelancet.com/journals/lancet/issue/current
Pandemic preparedness and COVID-19: an exploratory analysis of infection and fatality rates, and contextual factors associated with preparedness in 177 countries, from Jan 1, 2020, to Sept 30, 2021
COVID-19 National Preparedness Collaborators
Open Access
The Lancet
Apr 16, 2022 Volume 399 Number 10334 p1441-1572, e37
https://www.thelancet.com/journals/lancet/issue/current
Estimating excess mortality due to the COVID-19 pandemic: a systematic analysis of COVID-19-related mortality, 2020–21
COVID-19 Excess Mortality Collaborators
Open Access
Nature
Volume 604 Issue 7905, 14 April 2022
https://www.nature.com/nature/volumes/604/issues/7905
Analysis | 06 April 2022 | Open Access
A joint NCBI and EMBL-EBI transcript set for clinical genomics and research
Matched Annotation from NCBI and EMBL-EBI (MANE) delivers joint transcript sets from Ensembl/GENCODE and RefSeq for standardizing variant reporting in clinical genomics and research.
Joannella Morales, Shashikant Pujar, Terence D. Murphy
Nature Biotechnology
Volume 40 Issue 4, April 2022
https://www.nature.com/nbt/volumes/40/issues/4
Editorial | 04 April 2022
Licensing for profit and for good
The Broad Institute’s enlightened licensing approach to CRISPR–Cas9 intellectual property stands out in the otherwise regrettable spat for patent rights over the foundational technology.
Nature Genetics
Volume 54 Issue 4, April 2022
https://www.nature.com/ng/volumes/54/issues/4
Article | 11 April 2022
Expanded COVID-19 phenotype definitions reveal distinct patterns of genetic association and protective effects
GWASs based on self-reported phenotypes in 736,723 individuals show distinct associations between risk loci and eight COVID-19 outcomes, suggesting differences in genetic susceptibility to infection upon exposure and severe and symptomatic disease.
Genevieve H. L. Roberts, Raghavendran Partha, Kristin A. Rand
Nature Genetics
Volume 54 Issue 4, April 2022
https://www.nature.com/ng/volumes/54/issues/4
Analysis | 28 March 2022 | Open Access
Global landscape of SARS-CoV-2 genomic surveillance and data sharing
Analyses on the global diversity of SARS-CoV-2 genomic surveillance across 118 countries and the extent of public availability of genomic data provide evidence to better inform SARS-CoV-2 surveillance policy.
Zhiyuan Chen, Andrew S. Azman, Hongjie Yu
New England Journal of Medicine
April 14, 2022 Vol. 386 No. 15
https://www.nejm.org/toc/nejm/medical-journal
Perspective
From Resentment to Reconnection — Reflections on Caring for the Unvaccinated A.C. Garfinkel
PLoS Biology
https://journals.plos.org/plosbiology/
(Accessed 16 Apr 2022)
Biosecurity in an age of open science
James Andrew Smith, Jonas B. Sandbrink
Essay | published 14 Apr 2022 PLOS Biology
https://doi.org/10.1371/journal.pbio.3001600
Abstract
The risk of accidental or deliberate misuse of biological research is increasing as biotechnology advances. As open science becomes widespread, we must consider its impact on those risks and develop solutions that ensure security while facilitating scientific progress. Here, we examine the interaction between open science practices and biosecurity and biosafety to identify risks and opportunities for risk mitigation. Increasing the availability of computational tools, datasets, and protocols could increase risks from research with misuse potential. For instance, in the context of viral engineering, open code, data, and materials may increase the risk of release of enhanced pathogens. For this dangerous subset of research, both open science and biosecurity goals may be achieved by using access-controlled repositories or application programming interfaces. While preprints accelerate dissemination of findings, their increased use could challenge strategies for risk mitigation at the publication stage. This highlights the importance of oversight earlier in the research lifecycle. Preregistration of research, a practice promoted by the open science community, provides an opportunity for achieving biosecurity risk assessment at the conception of research. Open science and biosecurity experts have an important role to play in enabling responsible research with maximal societal benefit.
PLoS One
http://www.plosone.org/
[Accessed 16 Apr 2022]
Research Article
A scholarly network of AI research with an information science focus: Global North and Global South perspectives
Kai-Yu Tang, Chun-Hua Hsiao, Gwo-Jen Hwang
Research Article | published 15 Apr 2022 PLOS ONE
https://doi.org/10.1371/journal.pone.0266565
Revista Panamericana de Salud Pública/Pan American Journal of Public Health (RPSP/PAJPH)
https://www.paho.org/journal/en
Selected Articles
Realistic evaluation mechanisms in primary health care interventions in rural and marginal urban populations
Review | Spanish |
13 Apr 2022
Revista Panamericana de Salud Pública/Pan American Journal of Public Health (RPSP/PAJPH)
https://www.paho.org/journal/en
Selected Articles
Realistic evaluation mechanisms in primary health care interventions in rural and marginal urban populations
Review | Spanish |
13 Apr 2022
Revista Panamericana de Salud Pública/Pan American Journal of Public Health (RPSP/PAJPH)
https://www.paho.org/journal/en
Geospatial models for SARS-CoV-2 outbreak control in Cartagena and Barranquilla, Colombia, 2020
Original research | Spanish |
13 Apr 2022
Revista Panamericana de Salud Pública/Pan American Journal of Public Health (RPSP/PAJPH)
https://www.paho.org/journal/en
Scientific publication speed and retractions of COVID-19 pandemic original articles
Original research | English |
13 Apr 2022
Revista Panamericana de Salud Pública/Pan American Journal of Public Health (RPSP/PAJPH)
https://www.paho.org/journal/en
Management and impact of interventions to reduce COVID-19 cases in Costa Rica
Original research | Spanish |
13 Apr 2022
Risk Management and Healthcare Policy
https://www.dovepress.com/risk-management-and-healthcare-policy-archive56
[Accessed 16 Apr 2022]
Original Research
Acceptance of COVID-19 Vaccine Among High-Risk Occupations in a Port City of China and Multifaceted Strategies for Increasing Vaccination Coverage: A Cross-Sectional Study
Sun Y, Li B, Li N, Li B, Chen P, Hao F, Sun C
Risk Management and Healthcare Policy 2022, 15:643-655
Published Date: 14 April 2022
Science
Volume 376| Issue 6590| 15 Apr 2022
https://www.science.org/toc/science/current
Policy Forum
Getting genetic ancestry right for science and society
BY Anna C. F. Lewis, et al.
14 Apr 2022: 250-252
We must embrace a multidimensional, continuous view of ancestry and move away from continental ancestry categories
Science Translational Medicine
Volume 14| Issue 640| 13 Apr 2022
https://www.science.org/toc/stm/current
Reviews
Point-of-care diagnostic tests for tuberculosis disease
BY Jia Mei Hong, et al.
06 Apr 2022
Advances in biomarkers and microfluidics technology facilitate the development of TB disease diagnostic point-of-care tests.
Vaccine
Volume 40, Issue 13 Pages 1913-2122 (18 March 2022)
https://www.sciencedirect.com/journal/vaccine/vol/40/issue/13
Review article Open access
Epidemiology and economic burden of meningococcal disease in Germany: A systematic review
S. Gruhn, J. Witte, W. Greiner, O. Damm, … M. Knuf
Pages 1932-1947
Vaccine
Volume 40, Issue 13 Pages 1913-2122 (18 March 2022)
https://www.sciencedirect.com/journal/vaccine/vol/40/issue/13
Research article Open access
Long-term effectiveness of human papillomavirus vaccines among adult women: A real-world scenario
Ga Young Lee, Perapong Inthasorn, Piyawat Laowahutanont, Saranath Lawpoolsri, … Punnee Pitisuttithum
Pages 1968-1976
Vaccine
Volume 40, Issue 13 Pages 1913-2122 (18 March 2022)
https://www.sciencedirect.com/journal/vaccine/vol/40/issue/13
Research article Full text access
Promoting immunization equity in Latin America and the Caribbean: Case studies, lessons learned, and their implication for COVID-19 vaccine equity
Isabella L. Chan, Robin Mowson, Juan Pedro Alonso, Javier Roberti, … Martha Velandia-González
Vaccine
Volume 40, Issue 13 Pages 1913-2122 (18 March 2022)
https://www.sciencedirect.com/journal/vaccine/vol/40/issue/13
Research article Open access
Impact of the Sinopharm’s BBIBP-CorV vaccine in preventing hospital admissions and death in infected vaccinees: Results from a retrospective study in the emirate of Abu Dhabi, United Arab Emirates (UAE)
Farida Ismail AlHosani, Anderson Eduardo Stanciole, Bashir Aden, Andrey Timoshkin, … Farah Mustafa
Vaccine
Volume 40, Issue 13 Pages 1913-2122 (18 March 2022)
https://www.sciencedirect.com/journal/vaccine/vol/40/issue/13
Research article Open access
Unmet need for COVID-19 vaccination coverage in Kenya
Samuel K. Muchiri, Rose Muthee, Hellen Kiarie, Joseph Sitienei, … Victor A. Alegana
Pages 2011-2019
Vaccine
Volume 40, Issue 13 Pages 1913-2122 (18 March 2022)
https://www.sciencedirect.com/journal/vaccine/vol/40/issue/13
Research article Full text access
The correlates and dynamics of COVID-19 vaccine-specific hesitancy
Eric Merkley, Peter John Loewen
Pages 2020-2027
Vaccine
Volume 40, Issue 13 Pages 1913-2122 (18 March 2022)
https://www.sciencedirect.com/journal/vaccine/vol/40/issue/13
Research article Full text access
Public perceptions and the willingness to get vaccinated against COVID-19: Lessons from Israel
Oren Heller, Yung Chun, Yaniv Shlomo, Ateret Gewirtz-Meydan, … Michal Grinstein-Weiss
Vaccine
Volume 40, Issue 13 Pages 1913-2122 (18 March 2022)
https://www.sciencedirect.com/journal/vaccine/vol/40/issue/13
Research article Open access
Conspiracy theories and misinformation about COVID-19 in Nigeria: Implications for vaccine demand generation communications
Chizoba Wonodi, Chisom Obi-Jeff, Funmilayo Adewumi, Somto Chloe Keluo-Udeke, … Ruth Faden
Pre-Print Servers
Gates Open Research
https://gatesopenresearch.org/browse/articles
Open Letter metrics AWAITING PEER REVIEW
Women’s groups and COVID-19: An evidence review on savings groups in Africa [version 1; peer review: awaiting peer review]
Olayinka Adegbite, Leigh Anderson, Sybil Chidiac, Osasuyi Dirisu, Jenna Grzeslo, Julia Hakspiel, Chinmaya Holla, Emily Janoch, Krishna Jafa, Shubha Jayaram, Grace Majara, Tabitha Mulyampiti, Eve Namisango, Eva Noble, Bukola Onyishi, David Panetta, Garima Siwach, Munshi Sulaiman, Rebecca Walcott, Sapna Desai, Thomas de Hoop
Peer Reviewers Invited
Funder
The Bill and Melinda Gates Foundation
PUBLISHED 12 Apr 2022
medRxiv
https://www.medrxiv.org/content/about-medrxiv
medRxiv is a free online archive and distribution server for complete but unpublished manuscripts (preprints) in the medical, clinical, and related health sciences. Preprints are preliminary reports of work that have not been certified by peer review. They should not be relied on to guide clinical practice or health-related behavior and should not be reported in news media as established information. medRxiv is for the distribution of preprints – complete but unpublished manuscripts – that describe human health research conducted, analyzed, and interpreted according to scientific principles…
COVID-19 vaccine coverage among immigrants and refugees in Alberta: a population-based cross-sectional study
Shannon E MacDonald, Yuba Raj Paudel, Crystal Du
medRxiv 2022.04.11.22273644; doi: https://doi.org/10.1101/2022.04.11.22273644
Can We Really Trust the Findings of the COVID-19 Research? Quality Assessment of Randomized Controlled Trials Published on COVID-19
Athira S Joshy, Christy Thomas, Saphal Surendran, Krishna Undela
medRxiv 2022.04.15.22273881; doi: https://doi.org/10.1101/2022.04.15.22273881
Mondo: Unifying diseases for the world, by the world
Nicole A Vasilevsky, Nicolas A Matentzoglu, Sabrina Toro, Joe E Flack, Harshad Hegde, Deepak R Unni, Gioconda Alyea, Joanna S Amberger, Larry Babb, James P Balhoff, Taylor I Bingaman, Gully A Burns, Tiffany J Callahan, Leigh C Carmody, Lauren E Chan, George S Chang, Michel Dumontier, Laura E Failla, May J Flowers, H A Garrett Jr., Dylan Gration, Tudor Groza, Marc Hanauer, Nomi L Harris, Ingo Helbig, Jason A Hilton, Daniel S Himmelstein, Charles T Hoyt, Megan S Kane, Sebastian Kohler, David Lagorce, Martin Larralde, Antonia Lock, Irene Lopez Santiago, Donna R Maglott, Adriana J Malheiro, Birgit HM Meldal, Julie A McMurry, Moni Munoz-Torres, Tristan H Nelson, David Ochoa, Tudor I Oprea, David Osumi-Sutherland, Helen Parkinson, Zoe M Pendlington, Ana Rath, Heidi L Rehm, Lyubov Remennik, Erin R Riggs, Paola Roncaglia, Justyne E Ross, Marion F Shadbolt, Kent A Shefchek, Morgan N Similuk, Nicholas Sioutos, Rachel Sparks, Ray Stefancsik, Ralf Stephan, Doron Stupp, Jagadish Chandrabose Sundaramurthi, Imke Tammen, Courtney L Thaxton, Eloise Valasek, Alex H Wagner, Danielle Welter, Patricia L Whetzel, Lori L Whiteman, Valerie Wood, Colleen H Xu, Andreas Zankl, Xingmin A Zhang, Christopher G Chute, Peter N Robinson, Christopher J Mungall, Ada Hamosh, Melissa A Haendel
medRxiv 2022.04.13.22273750; doi: https://doi.org/10.1101/2022.04.13.22273750
There are thousands of distinct disease entities and concepts, each of which are known by different and sometimes contradictory names. The lack of a unified system for managing these entities poses a major challenge for both machines and humans that need to harmonize information to better predict causes and treatments for disease. The Mondo Disease Ontology is an open, community-driven ontology that integrates key medical and biomedical terminologies, supporting disease data integration to improve diagnosis, treatment, and translational research. Mondo records the sources of all data and is continually updated, making it suitable for research and clinical applications that require up-to-date disease knowledge.
Triangulating evidence in health sciences with Annotated Semantic Queries
Yi Liu, Tom R Gaunt
medRxiv 2022.04.12.22273803; doi: https://doi.org/10.1101/2022.04.12.22273803
Profile of Brazilian inpatients with COVID-19 vaccine breakthrough infection and risk factors for unfavorable outcome
Matheus Alexandre Santos de Jesus, Natália Satchiko Hojo-Souza, Thiago Rocha de Moraes, Daniel Ludovico Guidoni, Fernanda Sumika Hojo Souza
medRxiv 2022.04.12.22273589; doi: https://doi.org/10.1101/2022.04.12.22273589
Effectiveness of COVID-19 vaccines against hospitalization and death in Canada: A multiprovincial test-negative design study
Sharifa Nasreen, Yossi Febriani, Héctor Alexander Velásquez García, Geng Zhang, Mina Tadrous, Sarah A. Buchan, Christiaan H. Righolt, Salaheddin M. Mahmud, Naveed Zafar Janjua, Mel Krajden, Gaston De Serres, Jeffrey C. Kwong
medRxiv 2022.04.13.22273825; doi: https://doi.org/10.1101/2022.04.13.22273825
Health Economic Burden of COVID-19 in Saudi Arabia
Khalidah A. Alenzi, Hamdan S. Al-malky, Ali F. Altebainawi, Hamidah Q. Abushomi, Fahad O. Alatawi, Moosa H. Atwadi, Moosa A. Khobrani, Dlal A. Almazrou, Nariman Alrubeh, Zainab A. Alsoliabi, Abdulaziz M. Kardam, Shakr A. Alghamdi, Abdulrahman Alasiri, Thamir M. Alshammari
medRxiv 2022.04.08.22273439; doi: https://doi.org/10.1101/2022.04.08.22273439
Effectiveness of the BNT162b vaccine fourth dose in reducing SARS-CoV-2 infection among healthcare workers in Israel, a multi-center cohort study
Matan J Cohen, Yonatan Oster, Allon E Moses, Avishay Spitzer, Shmuel Benenson, the Israeli-hospitals 4th vaccine Working Group
medRxiv 2022.04.11.22273327; doi: https://doi.org/10.1101/2022.04.11.22273327
The relation between COVID-19 vaccinations and public governance to improve preparedness of next pandemic impacts and crisis management: a global study
Mario Coccia, Igor Benati
medRxiv 2022.04.10.22273663; doi: https://doi.org/10.1101/2022.04.10.22273663
Abstract
The goal of this study is to analyze the relationship between COVID-19 vaccinations and public governance performing a global analysis of more than 110 countries worldwide. Methodology applies the Independent Samples T-Test that compares the means of two independent groups (countries with high/low level of vaccinations) to determine whether there is statistical evidence that the associated population means of indicators of public governance are significantly different. Findings suggest that high levels of governance can support a better function of health systems in the rollout of vaccinations to cope with COVID-19 pandemic crisis. This study may assist long-run policy of governments to improve good governance and health systems of countries in order to reinforce the preparedness to face next pandemic threats and in general future crisis management in society.
Economic vulnerability and poor service delivery made it more difficult for shack-dwellers to comply with COVID-19 restrictions: The impracticability and inequitable burden of universal/unstratified public health policies
GTH Ellison, RB Mattes, H Rhoma, T De Wet
medRxiv 2022.04.07.22273499; doi: https://doi.org/10.1101/2022.04.07.22273499
COVID-19 vaccine for people who live and work in prisons worldwide: A scoping review
Nasrul Ismail, Lara Tavoschi, Babak Moazen, Alicia Roselló, Emma Plugge
medRxiv 2022.04.07.22273414; doi: https://doi.org/10.1101/2022.04.07.22273414
Novel Privacy Considerations for Large Scale Proteomics
Andrew C. Hill, Elizabeth M. Litkowski, Ani Manichaikul, Leslie Lange, Katherine A. Pratte, Katerina J. Kechris, Matthew DeCamp, Marilyn Coors, Victor E. Ortega, Stephen S. Rich, Jerome I. Rotter, Robert E. Gerzsten, Clary B. Clish, Jeffery Curtis, Xiaowei Hu, Debby Ngo, Wanda K O’Neal, Deborah Meyers, Eugene Bleecker, Brian D. Hobbs, Michael H. Cho, Farnoush Banaeikashani, Russell P. Bowler
medRxiv 2022.04.06.22269907; doi: https://doi.org/10.1101/2022.04.06.22269907
COVID-19 Vaccination Mandates and Vaccine Uptake
Alexander Karaivanov, Dongwoo Kim, Shih En Lu, Hitoshi Shigeoka
medRxiv 2021.10.21.21265355; doi: https://doi.org/10.1101/2021.10.21.21265355 Revision
Acceptance of and preference for COVID-19 vaccination in healthcare workers: a comparative analysis and discrete choice experiment
Chuanxi Fu, Zheng wei, Fengchang Zhu, Sen Pei, Shunping Li, Liuren Zhang, Xiaohui Sun, Yue Wu, Ping Liu, Mark Jit
medRxiv 2020.04.09.20060103; doi: https://doi.org/10.1101/2020.04.09.20060103 Revision
SARS-CoV-2 reinfections with BA.1 (Omicron) variant among fully vaccinated individuals in the northeast of Brazil
Francisco P. F. Neto, Diego G. Teixeira, Dayse C. S. da Cunha, Ingryd C. Morais, Celisa P. M. Tavares, Genilson P. Gurgel, Sanderson D. do Nascimento, David C. dos Santos, Alexandre de O. Sales, Selma M.B. Jerônimo
medRxiv 2022.04.08.22272726; doi: https://doi.org/10.1101/2022.04.08.22272726
Wellcome Open Research [to 16 Apr 2022]
https://wellcomeopenresearch.org/browse/articles
[Accessed 16 Apr 2022]
Wellcome Open Research provides all Wellcome researchers with a place to rapidly publish any results they think are worth sharing. All articles benefit from rapid publication, transparent peer review and editorial guidance on making all source data openly available.
Data Note metrics AWAITING PEER REVIEW
An open dataset of Plasmodium vivax genome variation in 1,895 worldwide samples [version 1; peer review: awaiting peer review]
MalariaGEN, Ishag Adam, Mohammad Shafiul Alam, Sisay Alemu, Chanaki Amaratunga, Roberto Amato, Voahangy Andrianaranjaka, Nicholas M Anstey, Abraham Aseffa, Elizabeth Ashley, Ashenafi Assefa, Sarah Auburn, Bridget E Barber, Alyssa Barry, Dhelio Batista Pereira, Jun Cao, Nguyen Hoang Chau, Kesinee Chotivanich, Cindy Chu, Arjen M. Dondorp, Eleanor Drury, Diego F. Echeverry, Berhanu Erko, Fe Espino, Rick Fairhurst, Abdul Faiz, María Fernanda Villegas, Qi Gao, Lemu Golassa, Sonia Goncalves, Matthew J Grigg, Yaghoob Hamedi, Tran Tinh Hien, Ye Htut, Kimberly J Johnson, Nadira Karunaweera, Wasif Khan, Srivicha Krudsood, Dominic P Kwiatkowski, Marcus Lacerda, Benedikt Ley, Pharath Lim, Yaobao Liu, Alejandro Llanos-Cuentas, Chanthap Lon, Tatiana Lopera-Mesa, Jutta Marfurt, Pascal Michon, Olivo Miotto, Rezika Mohammed, Ivo Mueller, Chayadol Namaik-larp, Paul N Newton, Thuy-Nhien Nguyen, Francois Nosten, Rintis Noviyanti, Zuleima Pava, Richard D Pearson, Beyene Petros, Aung P Phyo, Ric N Price, Sasithon Pukrittayakamee, Awab Ghulam Rahim, Milijaona Randrianarivelojosia, Julian C Rayner, Angela Rumaseb, Sasha V Siegel, Victoria J Simpson, Kamala Thriemer, Alberto Tobon-Castano, Hidayat Trimarsanto, Marcelo Urbano Ferreira, Ivan D Vélez, Sonam Wangchuk, Thomas E Wellems, Nicholas J White, Timothy William, Maria F Yasnot, Daniel Yilma
Peer Reviewers Invited
Funders
Wellcome Trust
Medical Research Council UK and the Department for International Development (DFID)
Bill and Melinda Gates Foundation
PUBLISHED 14 Apr 2022
Research Article metrics
Revised
Stage 2 Registered Report: How responsibility attributions to self and others relate to outcome ownership in group decisions [version 2; peer review: 2 approved]
Matt Jaquiery, Marwa El Zein
Peer Reviewers John A. Dewey; Nura Sidarus
Funders
Wellcome Trust
Medical Research Council
University of Oxford
LATEST VERSION PUBLISHED 11 Apr 2022
Research Article metrics
Revised
A survey to gather perspectives of DBT/Wellcome Trust India Alliance-funded researchers on public engagement with science [version 2; peer review: 2 approved]
Sarah Iqbal, Banya Kar
Peer Reviewers Marina Joubert; Mary Chambers and Han Dong Thai Tran
Funder
DBT/Wellcome Trust India Alliance
LATEST VERSION PUBLISHED 08 Apr 2022
Think Tanks
Brookings
http://www.brookings.edu/
Accessed 16 Apr 2022
[No new digest content identified]
Center for Global Development [to 16 Apr 2022]
https://www.cgdev.org/
Research [Selected]
POLICY PAPERS
Beyond India’s Lockdown: PMGKY Benefits During the COVID-19 Crisis and the State of Digital Payments
Alan Gelb et al.
April 11, 2022
India imposed a lock-down in response to the COVID-19 pandemic in March 2020 and began a gradual re-opening in June. A telephonic survey in April examined the early effectiveness of information and the massive PMGKY social protection program (Policy Paper 217). This paper analyzes a second-round sur…
Chatham House [to 16 Apr 2022]
https://www.chathamhouse.org/
Accessed 16 Apr 2022
[No new digest content identified]
CSIS
https://www.csis.org/
Accessed 16 Apr 2022
Upcoming Event
Equity in Immunization Services to Ensure “A Long Life for All”
April 28, 2022
Upcoming Event
Innovation and IP’s Role in Combatting the Covid-19 Pandemic
April 27, 2022
Upcoming Event
AMR as a Global Security Threat: Destabilizing Food Systems and Healthy Communities
April 25, 2022
Commentary
Removing Patent Rights to Lower Drug Costs Is a Dangerous Precedent
April 14, 2022 | By Walter G. Copan
Kaiser Family Foundation
https://www.kff.org/search/?post_type=press-release
Accessed 16 Apr 2022
Accessed 16 Apr 2022
[No new digest content identified]
ODI [Overseas Development Institute] [to 16 Apr 2022]
https://odi.org/en/publications/
Publications
Accessed 16 Apr 2022
[No new digest content identified]
Rand [to 16 Apr 2022]
https://www.rand.org/pubs.html
Reports, Selected Journal Articles
Report
How Extremism Operates Online: A Primer
In this Perspective, the second in a RAND Corporation series on online extremist material, the authors explore how the internet affects extremist activities and how exposure to or consumption of such content influences the behavior of internet users.
Apr 12, 2022
Alexandra T. Evans, Heather J. Williams
Vaccines and Global Health: The Week in Review is a weekly digest summarizing news, events, announcements, peer-reviewed articles and research in the global vaccine ethics and policy space. Content is aggregated from key governmental, NGO, international organization and industry sources, key peer-reviewed journals, and other media channels. This summary proceeds from the broad base of themes and issues monitored by the Center for Vaccine Ethics & Policy in its work: it is not intended to be exhaustive in its coverage. You are viewing the blog version of our weekly digest, typically comprised of between 30 and 40 posts below all dated with the current issue date
.– Request an Email Summary: Vaccines and Global Health : The Week in Review is published as a single email summary, scheduled for release each Saturday evening before midnight (EDT in the U.S.). If you would like to receive the email version, please send your request to david.r.curry@centerforvaccineethicsandpolicy.org.
– pdf version: A pdf of the current issue is available here:
– blog edition: comprised of the approx. 35+ entries posted below.
– Twitter: Readers can also follow developments on twitter: @vaxethicspolicy.
.
– Links: We endeavor to test each link as we incorporate it into any post, but recognize that some links may become “stale” as publications and websites reorganize content over time. We apologize in advance for any links that may not be operative. We believe the contextual information in a given post should allow retrieval, but please contact us as above for assistance if necessary.
Support this knowledge-sharing service: Your financial support helps us cover our costs and to address a current shortfall in our annual operating budget. Click here to donate and thank you in advance for your contribution.
.
David R. Curry, MS
Executive Director
Center for Vaccine Ethics and Policy
GAVI COVAX Advance Market Commitment Summit 2022
One Third of Humanity Still Unvaccinated, Secretary-General Tells GAVI COVAX Advance Market Commitment Summit 2022, Citing ‘Deeply Unequal World’
8 April 2022
SG/SM/21233
Following is the text of UN Secretary-General António Guterres’ video message for the GAVI COVAX Advance Market Commitment Summit 2022, “One World Protected – Break COVID Now”, in New York today:
I commend the Government of Germany and GAVI for organizing this important Summit. This gathering is a critical reminder that the COVID-19 pandemic is far from over.
We’re seeing 1.5 million new cases each day. Large outbreaks are spreading in Asia. A new wave is sweeping across Europe. And some countries are reporting their highest death rates since the start of the pandemic.
Omicron is a startling reminder of how quickly COVID-19 can mutate and spread, especially in the absence of high vaccination coverage. Some high-income countries are preparing for their second booster doses. And yet one third of humanity remains unvaccinated. This is a brutal indictment of our deeply unequal world. It’s also a prime breeding ground for new variants, more deaths and increased human and economic misery. The next variant is not a question of “if”. It’s a question of “when”.
We are far from our target of every country reaching 70 per cent vaccination coverage by the middle of this year. And with new variants emerging every four months on average, time is of the essence. Supply is not the issue. Manufacturers are producing 1.5 billion doses per month. And thanks to its remarkable procurement, shipment and delivery system, the COVAX Facility and its Advance Market Commitment mechanism has managed to deliver 1.2 billion doses so far to countries in need.
This proves that progress is possible. But, the window is closing fast. Governments and pharmaceutical companies need to work better together to deliver vaccines to every person, everywhere — not just in wealthy countries. This means countries fulfilling and accelerating dose-sharing and donation commitments to COVAX with better quality of supply. And it means having strong national vaccinedelivery systems at the ready — including efforts to counter disinformation and get vaccines into arms.
I also call on countries to commit new funding for the ACT-Accelerator and COVAX this year. And we need to help all countries prepare for future pandemics by multiplying the number of countries able to locally produce tests, vaccines and treatments. So, we can save lives quickly and equitably when the next variant or pandemic strikes. And ultimately, build stronger health systems that are accessible to all.
Investments today will not only save lives. They will strengthen overwhelmed health systems for the future, and help all countries move closer to sustainable recovery. The COVID-19 pandemic is not over. But, it can be. Let’s end it together.
::::::
World leaders commit US$ 4.8 billion to help Break COVID Now
2022 Break COVID Now Summit co-hosted by Gavi, alongside Germany (G7 President), Ghana, Indonesia (G20 President), and Senegal (African Union chair) reaffirms support for COVAX and global solidarity in fighting the COVID-19 pandemic
Summit sees US$ 4.8 billion in commitments, securing vital cash to help lower-income countries boost vaccinations now, secure equitable access for new vaccine procurements plus additional help for countries looking to procure their own vaccines in the future. Indonesia’s President, leading the G20 this year, extended his support for the launch of the Pandemic Vaccine Pool as a key instrument for response and resiliency.
Professor José Manuel Barroso, Chair of the Gavi Board, said: “We welcome this incredible show of global solidarity from so many stakeholders at a time when the world faces multiple challenges.”
Geneva, 8 April 2022 – The 2022 Break COVID Now Summit, co-hosted by Gavi, the Vaccine Alliance alongside the governments of Germany, Ghana, Indonesia and Senegal saw world leaders come together to reaffirm support for equitable access to COVID-19 vaccines and acting urgently to break COVID now.
With Germany, Indonesia and Senegal holding Presidencies in the G7, G20 and AU respectively, the Summit represented an affirmation of the international community’s support for COVAX’s multilateral approach to vaccine equity. The event also successfully secured commitments valued at US$ 4.8 billion for the Gavi COVAX Advance Market Commitment (AMC), the mechanism which supports equitable access to COVID-19 vaccines for lower-income countries.
These commitments mean a total of US$ 1.7 billion in new sovereign donor pledges towards the 2022 AMC fundraising ask, as well as US$ 2.1 billion worth of commitments via new innovative financial mechanisms provided by the EIB and the United States Development Finance Corporation (DFC), and least US$ 1 billion made available by three multilateral development banks (MDBs) – World Bank, Asian Development Bank and European Investment Bank (EIB).
The commitments made will enable COVAX to provide urgent delivery support for lower-income countries and ensure dose donations can be shipped and administered. They have also enabled Gavi to launch the Pandemic Vaccine Pool to support future procurement of new COVID-19 vaccines, on behalf of COVAX AMC participants, should they be needed. Commitments from MDBs enable low-cost financing for these countries to purchase additional vaccines beyond those that are donor-funded.
New donor commitments made to the Gavi COVAX AMC include:
Japan, co-host of the 2021 AMC Summit, pledged US$ 500 million
Germany, co-host of today’s Break COVID Now Summit, pledged EUR 400 million
Canada pledged CAD 220 million
Brazil pledged US$ 86.7 million
European Commission EUR 75 million
Australia pledged AUD 85 million
Finland pledged EUR 2 million
Iceland pledged ISK 250 million
Luxembourg pledged EUR 1 million
Vietnam pledged US$ 500,000
Regional Government of Catalonia, Spain pledged EUR 290’000
Provincial Council of Bizkaia, Spain pledged EUR 100’000
Estonia pledged EUR 40,000
Malta pledged EUR 40,000
Workday Foundation pledged US$ 300,000
CODE(RED) Campaign pledged US$ 200,000
Other donors also pledged a total of US$ 122.6 million
The pledges made today build on top of the commitments made at the 2022 One World Protected event, which launched the 2022 AMC Investment Opportunity on 19 January.
A number of countries and groups expressed intention to make additional contributions to COVAX, including in support of the Pandemic Vaccine Pool, but were unable to announce today due to their budgetary processes and the short timeline of this emergency appeal…
IMF Working Paper
A Global Strategy to Manage the Long-Term Risks of COVID-19
Authors/Editors: Ruchir Agarwal ; Gita Gopinath ; Jeremy Farrar ; Richard Hatchett ; Peter Sands
Publication Date: April 5, 2022 :: 26 pages
Electronic Access: Free Download.
Summary:
The pandemic is not over, and the health and economic losses continue to grow. It is now evident that COVID-19 will be with us for the long term, and there are very different scenarios for how it could evolve, from a mild endemic scenario to a dangerous variant scenario. This realization calls for a new strategy that manages both the uncertainty and the long-term risks of COVID-19. There are four key policy implications of such as strategy.
First, we need to achieve equitable access beyond vaccines to encompass a comprehensive toolkit.
Second, we must monitor the evolving virus and dynamically upgrade the toolkit.
Third, we must transition from the acute response to a sustainable strategy toward COVID-19, balanced and integrated with other health and social priorities.
Fourth, we need a unified risk-mitigation approach to future infectious disease threats beyond COVID-19.
Infectious diseases with pandemic potential are a threat to global economic and health security. The international community should recognize that its pandemic financing addresses a systemic risk to the global economy, not just the development need of a particular country. Accordingly, it should allocate additional funding to fight pandemics and strengthen health systems both domestically and overseas. This will require about $15 billion in grants this year and $10 billion annually after that.
Series: Working Paper No. 2022/068
Disclaimer: IMF Working Papers describe research in progress by the author(s) and are published to elicit comments and to encourage debate. The views expressed in IMF Working Papers are those of the author(s) and do not necessarily represent the views of the IMF, its Executive Board, or IMF management.
New IMF Staff Paper Strategy to Manage the Long-Term Risks of COVID-19
April 5, 2022
Washington, DC: The International Monetary Fund, in partnership with the Coalition for Epidemic Preparedness Innovations (CEPI), the Global Fund, and Wellcome Trust published today “A Global Strategy to Manage the Long-term Risks of COVID-19” working paper, which calls for a more comprehensive and integrated pandemic response from the international community.
In the joint paper, the four global organizations assert that ending the pandemic everywhere remains an urgent economic, health, and moral priority for the world—advocating for the following:
Gita Gopinath, First Deputy Managing Director, IMF:
“It is now clear that COVID-19 is likely to be with us for the long-term. Given the many possible scenarios for the evolution of COVID-19 (from benign to severe scenarios), and given the limited resources countries have, we need a new strategy.
“Countries need a more comprehensive COVID-19 toolkit for fighting the pandemic that includes vaccines, tests, treatments – and bolstering the resilience of health systems so they are in a better position to tackle both COVID-19 and other deadly diseases in a sustainable, effective way.
“Overall, health security is economic security. As recently as our January World Economic Outlook Update, we’d estimated the cumulative losses from the pandemic to reach $13.8 trillion. The international community should recognize that its pandemic financing addresses a systemic risk to the global economy. Thus, we are calling for additional funding to fight pandemics and to strengthen health systems. This will require about $15 billion in grants this year and $10 billion annually after that. The cost of inaction – for all of us – is very high. We need to act – now.
“Together with our partners on the Multilateral Leaders Taskforce and with the ACT Accelerator, the IMF stands ready to help countries meet the challenges of the pandemic and their financing needs—including through a Resilience and Sustainability Trust (RST).”
Richard Hatchett, CEO, CEPI:
“In many ways COVID-19 has shown us the potential of human ingenuity and innovation when we direct out energy and resources in fighting a common enemy. It has also tragically thrown into sharp focus a global failure to work multilaterally to ensure equal access to life saving vaccines.
“Vaccines are, and will continue to be, at the forefront of how modern societies counter infectious disease threats. They are one of our most potent tools against pandemic risks and will be critical to any future response. But if they are to truly fulfill their potential in preventing future pandemics their development must also go alongside investments in global surveillance, R&D, manufacturing, and health systems.
“During the course of this pandemic we have seen scientific advances that may well have taken decades to make in ‘peacetime’. Now, more than ever, the world now has the tools, the platforms, the concepts, and the experience to build a system that can more effectively meet the threat of future viruses.
“A future in which we are to respond to the next Disease X with new vaccines, therapeutics, and diagnostics in just 100 days is possible—but it will take vision, political will, and commensurate financial investments from governments around the world. We estimate that it will cost the world around $10 billion a year to ensure adequate global pandemic preparedness: this price tag is substantial, but failure to invest now—and build on the gains made in the response to COVID-19—will result in human and economic costs that will reverberate for generations.”
Peter Sands, Executive Director, Global Fund:
“The next phase of the fight against COVID-19 will be different. We are in for a long fight against a virus that continues to evolve. So we must shift to a more sustainable response that recognizes the linkages between responding to COVID-19, tackling the earlier pandemics of HIV, TB and malaria, and preparing for future pandemic threats. We should step up investment in systems for health, intentionally maximizing the synergies between interventions against existing infectious diseases and initiatives to prevent, detect and respond to future infectious disease outbreaks. Stronger and more resilient systems for health, including community systems, will enable us to protect everyone, everywhere from the deadliest infectious diseases.”
Jeremy Farrar, Director, Wellcome Trust:
“These last two years have shown that remarkable progress is possible when the world comes together and supports science boldly at scale, across borders. This approach gave us lifesaving Covid-19 vaccines and treatments in record time. Now is not the time to ease up – the virus’s next move is anything but certain and the risk of new variants high. We all desperately want this pandemic to be over. But simply wishing for the most optimistic scenario won’t make it so.
“We need to set our sights on developing next generation vaccines that can block transmission and won’t require endless boosters, strengthening genomic surveillance globally so we can identify and track new variants and improving global access to vaccines, treatments and tests. Leaving any countries unprotected, puts us all at risk.
“Most importantly, the response must be built on international co-operation. Only by working together can leaders achieve long-lasting and sustainable recovery from Covid-19 and prepare for the epidemic and pandemic threats of tomorrow.”
Ukraine

[Excerpts]
1.2 Access to health care in Ukraine
There are many challenges to accessing health care, with active hostilities and a lack of public transport restricting movement. Close to 1000 health facilities are in proximity to conflict areas or are in changed areas of control, multiple hospitals have been repurposed to care for wounds and half of the pharmacies in Ukraine have closed, which leaves the health system vulnerable to infrastructural damage and severe disruptions in critical services. As a consequence, there is limited or no access to medicines, health facilities, and health-care workers in some areas. Between 24 February and 6 April a total of 91 attacks1 on health care have been reported, resulting in 46 injuries and 73 deaths.2 Further attacks are being verified. Since 24 February 274 hospitals have been shelled, 13 have been completely destroyed, and 70 ambulances have been disabled by shelling…
Risk of emergence and spread of infectious diseases
Ongoing epidemics
The incidence of COVID-19 continues to decrease, with 14 120 new cases and 147 new deaths reported between 31 March and 5 April. However, these numbers should be interpreted carefully due to underreporting of COVID-19 cases and deaths. From 23 February to 6 April the seven-day average number of polymerase chain reaction tests and antigen-rapid diagnostic tests has declined significantly, with a 93% (from 42 460 to 1531) and 89% (from 51 484 to 6194) decrease, respectively. Vaccination uptake remains low, particularly in vulnerable populations, and the disruption in testing and treatment puts those most vulnerable at increased risk of severe illness and death.
Epidemic risk
:: Poor ventilation and overcrowding increase the risk of spread of respiratory infections, including COVID-19. Lack of access to water, sanitation and hygiene (WASH) heightens the risk of emergence of foodborne and waterborne diseases. According to United Nations Office for the Coordination of Humanitarian Affairs (OCHA), around six million people have either limited or no access to safe water, with active hostilities preventing repair teams from fixing damaged systems and restoring access to water while also hindering the delivery of water in the hardest-hit areas, like the Donetsk and Luhansk oblasts. Of note, an outbreak of cholera was reported in 2011 in the Mariupol region, while a single case was reported in 2016 in the Zaporizhzhya oblast, highlighting the risk of cholera outbreaks.
:: Suboptimal vaccination coverage for routine and childhood immunizations, including measles and poliomyelitis (polio), increases the risk of re-emergence and transmission of vaccine-preventable diseases. Notably, two cases of circulating vaccine-derived poliovirus type 2 (cVDPV2) were reported in Ukraine in 2021. On 1 February 2022 a national supplemental polio immunization campaign targeting nearly 140 000 children was launched, but due to the current situation it has been deprioritized and significantly slowed down.3
:: With the arrival of spring and rising temperatures, disrupted access to WASH and damage to homes may increase the risk of vector-borne diseases such as West Nile fever and tick-borne encephalitis.
AMR
AMR Action Fund Announces First Investments in Adaptive Phage Therapeutics and Venatorx Pharmaceuticals
Deals mark an important step toward AMR Action Fund’s goal of bringing two to four new antibiotics to market to take on growing threat of drug-resistant bacteria
APRIL 4, 2022, BOSTON, MA –The AMR Action Fund, the world’s largest public-private partnership investing in biotech companies that are developing antibiotics, announced today that it has invested in Adaptive Phage Therapeutics (APT) and Venatorx Pharmaceuticals. The transactions mark the Fund’s first investments and are an important step toward its goals of bringing to market new treatments for priority pathogens identified by the World Health Organization (WHO) and U.S. Centers for Disease Control and Prevention.
“From inception, the AMR Action Fund has focused on identifying investments that will yield urgently needed treatments and catalyze long-term innovation to take on the growing threat of antimicrobial resistance (AMR), which now kills more people annually than HIV/AIDS or malaria,” said Bill Burns, Board Chair of the AMR Action Fund. “Welcoming Adaptive Phage Therapeutics and Venatorx Pharmaceuticals as our first portfolio companies demonstrates that we are well on our way to fulfilling this important mission.”…
“Adaptive Phage Therapeutics and Venatorx Pharmaceuticals are poised to change the treatment landscape for drug-resistant infections and deliver significant benefit to patients,” said AMR Action Fund CEO, Henry Skinner. “While this is a major milestone for the Fund, our work is just beginning. We plan to commit over $100 million in capital this year in companies developing clinically differentiated antimicrobials with the potential to treat the most urgent unmet clinical needs, and we will continue investing in promising biotechs in the years ahead to ensure that patients around the world have the treatments they need in the ever-evolving fight against superbugs. Our investments are substantial, but we alone are not enough to take on the global challenge of AMR. It is now imperative that policymakers around the world enact market reforms to support investment in these urgently needed medications.”
APT’s approach leverages an ever-growing library of systematically discovered, selected, catalogued, and curated bacteriophages (phages), naturally occurring viruses that infect and kill bacteria, which collectively provide broad coverage against many of the world’s highest priority antibiotic-resistant bacteria. Phages from APT’s phage bank are matched to treat patients’ infections through a proprietary susceptibility assay, and are being tested on a range of infections, including prosthetic joint infections, bone infections (osteomyelitis), and lung infections. The AMR Action Fund’s investment in APT was executed as an extension to a Series B round led by Deerfield Management Company…
::::::
IFPMA – Largest pharmaceutical industry-backed collective venture fund tackling antimicrobial resistance announces first portfolio investments
April 4, 2022
::::::
Coronavirus [COVID-19] – WHO
Public Health Emergency of International Concern (PHEIC)
https://www.who.int/emergencies/diseases/novel-coronavirus-2019
Weekly Epidemiological and Operational updates
Last update: 08 Apr 2022
Confirmed cases :: 494 587 638
Confirmed deaths :: 6 170 283
Vaccine doses administered: 11 250 782 214
::::::
Weekly epidemiological update on COVID-19 – 5 April 2022
Overview
After the increase observed during the first half of March 2022, the number of new COVID-19 cases has decreased for a second consecutive week, with a 16% decline during the week of 28 March through 3 April 2022 as compared to the previous week.
The number of new weekly deaths also decreased sharply (-43%) as compared to the previous week, during which an artificial spike in deaths was observed.
Across the six WHO regions, over nine million new cases and over 26 000 new deaths were reported, and all the regions show decreasing trends both in the number of new weekly cases and new weekly deaths.
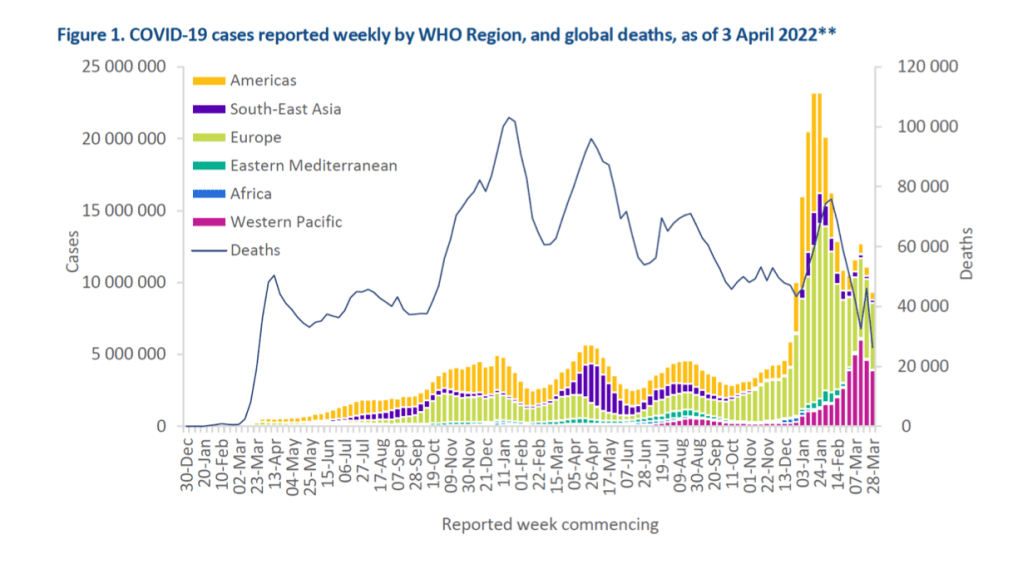
As of 3 April 2022, just over 489 million cases and over 6 million deaths have been reported globally.
In this edition, we provide an update on the geographic distribution of circulating SARS-CoV-2 variants of concern (VOCs), including the prevalence and summary of current evidence of the Omicron variant. We also provide updates on recombinants and vaccine effectiveness for the Delta and Omicron variants.
Over two-thirds of Africans exposed to virus which causes COVID-19: WHO study
07 April 2022
Brazzaville – Up to 65% of Africans have been infected by SARS-CoV-2, the virus which causes COVID-19, a World Health Organization (WHO) analysis finds. The study finds that true infections on the continent were 97 times larger than reported confirmed cases.
The analysis, which is available as a pre-print under peer review, synthesized 151 studies published on seroprevalence in Africa between January 2020 and December 2021. It found that exposure to SARS-CoV-2 skyrocketed from 3% (1.0-9.2% range) in June of 2020 to 65% (56.3-73% range) by September of 2021, or 800 million infections compared with 8.2 million cases reported at that time. The study showed that exposure to the virus rose sharply following the emergence of the Beta and the Delta variants.
The analysis revealed that the true number of infections could be as much as 97 times higher than the number of confirmed reported cases. This compares to the global average where true number of infections is 16 times higher than the number of confirmed reported cases.
However, seroprevalence varied widely within and across countries in Africa – higher in more dense urban areas than in less populated rural areas – and between age groups, with children aged 0-9 years having fewer infections compared with adults. Exposure to the virus also varied between countries and Africa’s sub-regions: seroprevalence appears to be highest in Eastern, Western and Central African regions.
The new analysis suggests that more than two-thirds of all Africans have been exposed to the COVID-19 virus. Globally seroprevalence studies have found a significant under-counting of cases occurring across the globe with 45.2% of the world’s population estimated to have been infected with the virus by September 2021. It is, however, difficult to compare figures for Africa with those of other regions, as many of the studies conducted cover different time periods.
The continent differentiates itself from other regions by its high number of asymptomatic cases, with 67% of cases having no symptoms.
“This analysis shows that current reported COVID-19 confirmed cases are only a fraction of the actual number of infections on the continent,” said Dr Matshidiso Moeti, WHO Regional Director for Africa. “This under-counting is occurring world-wide and it’s no surprise that the numbers are particularly large in Africa where there are so many cases with no symptoms.”…
Status of COVID-19 Vaccines within WHO EUL/PQ evaluation process 02 March 2022
[New additions; Full scale view available at title link above]
[Updated on 09 Apr 2022]

COVID Vaccines/Therapeutics – Developer/Manufacturer Announcements
[Selected press releases/announcements from organizations from WHO EUL/PQ listing above and other organizations]
AstraZeneca
Press Releases – No new digest announcements identified
Bharat Biotech
Press Releases – No new company announcements identified
Suspension of supply of COVID-19 vaccine (COVAXIN®)
2 April 2022
Today, WHO confirmed the suspension of supply of Covaxin (Bharat Biotech) through UN procurement agencies, and recommended that countries using the vaccine take action as appropriate.
The suspension is in response to the outcome of a WHO inspection on 14–22 March 2022, and the need to conduct process and facility upgrade to address recently identified deficiencies in good manufacturing practices (GMP).
Bharat Biotech has committed to addressing the GMP deficiencies and is developing a corrective and preventive action plan for submission to the Drugs Controller General of India (DCGI) and WHO. In the interim and as a precautionary measure, the company has indicated that it will suspend production of Covaxin for export. As a consequence, supply will be interrupted for the foreseeable future.
The risk assessment to date does not indicate change in the risk–benefit ratio. The data, available to WHO, indicate the vaccine is effective and no safety concerns exist…
BioCubaFarma – Cuba
Últimas Noticias – Website not leading at inquiry
Biological E
News
BE to Get mRNA Tech from WHO to Produce COVID-19 Vaccines
WHO selects BE as a recipient of mRNA technology
Biological E. Limited produces a number of critical & life-saving vaccines, including CORBEVAXTM
Hyderabad, April 4, 2022: Biological E. Limited (BE), one of the world’s largest vaccine manufacturers, has today announced that it has been selected as a recipient of mRNA technology from the World Health Organisation (WHO) technology transfer hub.
Biontech
Press Releases
BioNTech Granted Pandemic Preparedness Contract by German Federal Ministry of Health
MAINZ, Germany, April 8, 2022 — BioNTech SE (Nasdaq: BNTX) today announced that it is one of the companies in Germany to be granted a pandemic preparedness contract by the Federal Republic of Germany. The framework agreement is aimed at pandemic preparedness including manufacturing and supply of mRNA vaccines in emergency situations in Germany.
“There is growing evidence that viral pandemics will continue to pose a public health challenge for years. This contract with the German government will ensure significant supply of vaccine doses to address potential public health threats by 2027,” said Sean Marett, Chief Business and Chief Commercial Officer at BioNTech…
CanSinoBIO
News – Website not responding at inquiry
CIGB
Latest News – No new digest announcements identified
Cinagen
Recent News – No new digest announcements identified
Clover Biopharmaceuticals – China
News – No new digest announcements identified
Curevac [Bayer Ag – Germany]
News – No new digest announcements identified
Gamaleya National Center
Latest News and Events – See Russia below.
IMBCAMS, China
Home – Website not responding at inquiry
Janssen/JNJ
Press Releases
Apr 04, 2022 United States
World Health Organization Updated Emergency Use Listing Recommends Johnson & Johnson COVID-19 Vaccine for Booster Us
:: WHO supports vaccine for use as same-dose and ‘mix-and-match’ booster following a primary mRNA vaccine regimen
:: WHO also recommends extended shelf-life of up to 11 months for Johnson & Johnson COVID-19 vaccine when stored at 2 to 8 degrees Celsius (36 to 46 degrees Fahrenheit)
Medicago
Media – No new digest announcements identified
Moderna
Press Releases
07 April, 2022
IAVI and Moderna Partner to Tackle Broad Global Health Priorities Using mRNA for Vaccines and Antibodies
Nanogen
News – No new digest announcements identified
Novavax
Press Releases
Novavax and Serum Institute of India Receive Emergency Use Authorization for Novavax’ COVID-19 Vaccine in Thailand
Apr 8, 2022
Pfizer
Recent Press Releases
04.07.2022
Pfizer to Acquire ReViral and Its Respiratory Syncytial Virus Therapeutic Candidates
:: Proposed acquisition strengthens Pfizer’s capabilities in infectious disease research and development with a complementary strategy to help improve patient outcomes through treatment for respiratory syncytial virus (RSV) infections and prevent illness through vaccination
:: Expands Pfizer’s innovative anti-infective pipeline and utilizes the company’s R&D, manufacturing and commercialization expertise with the goal of addressing a significant unmet need for RSV treatments
R-Pharm
https://rpharm-us.com/index.php
[No news or media page identified]
Sanofi Pasteur
Press Releases – No new digest announcements identified
Serum Institute of India
NEWS & ANNOUNCEMENTS – No new digest announcements identified
Shifa Pharmed [Iran]
http://shafapharmed.com/
No news page identified.
Sinopharm/WIBPBIBP
News – No new digest announcements identified
Sinovac
Press Releases – No new digest announcements identified
Vector State Research Centre of Viralogy and Biotechnology
Home – No new digest announcements identified
WestVac Biopharma
Media – No new digest announcements identified
Zhifei Longcom, China
[Anhui Zhifei Longcom Biologic Pharmacy Co., Ltd.]
[No website identified]
::::::
GSK
Press releases for media – No new digest announcements identified
Merck
News releases
Expansion of Elkton, Virginia Manufacturing Facility to Further Increase Merck’s HPV Vaccine Supply and Support Broader and Equitable Access
April 04, 2022
KENILWORTH, N.J.–(BUSINESS WIRE)– Merck (NYSE: MRK), known as MSD outside the United States and Canada, reaffirmed its commitment to enable broad equitable access to the company’s HPV vaccines. To support this, the company has invested significantly in manufacturing, and recently expanded its vaccines manufacturing facility located in Elkton, VA, completing the construction of 120,000 square feet and adding 150 new jobs at the site to further increase capacity and supply of the company’s human papillomavirus (HPV) vaccines, following regulatory reviews and approvals…
Novartis
News – No new digest announcements identified
SK Biosciences
Press releases –
SK bioscience Submits Post Approval Change Application of Protein-Based COVID-19 Vaccine for Adolescent Authorization to KMFDS
2022. 04. 07
Valneva
Press Releases – No new digest announcements identified
UNICEF COVID-19 Vaccine Market Dashboard :: Agreements Table Accessed 09 Apr 2022
An overview of information collected from publicly announced bilateral and multilateral supply agreements [no new agreements since 10/22/2021 reported]

COVID-19 Global Targets and Progress Tracker – IMF
The COVID-19 Global Targets and Progress Tracker presents a consolidated view of the progress towards global COVID-19 targets, barriers in access to COVID-19 tools, and delivery of donor pledges.

The global targets presented in the Tracker are based on an alignment of the targets identified in the IMF Pandemic Proposal, ACT-A Strategic Plan & Budget, and the US-hosted Global C19 Summit, and as such have been reaffirmed by multilateral institutions and global leaders. We will continue to enhance the tracker as we improve our data collection efforts.
Global Dashboard on COVID-19 Vaccine Equity
The Dashboard is a joint initiative of UNDP, WHO and the University of Oxford with cooperation across the UN system, anchored in the SDG 3 Global Action Plan for Healthy Lives and Well-being for All.
Dashboard on Vaccine Equity [accessed 09 Apr 2022]: https://data.undp.org/vaccine-equity/
See also visualization on Vaccine Access and Vaccine Affordability

Global COVID-19 Access Tracker
https://www.covid19globaltracker.org/
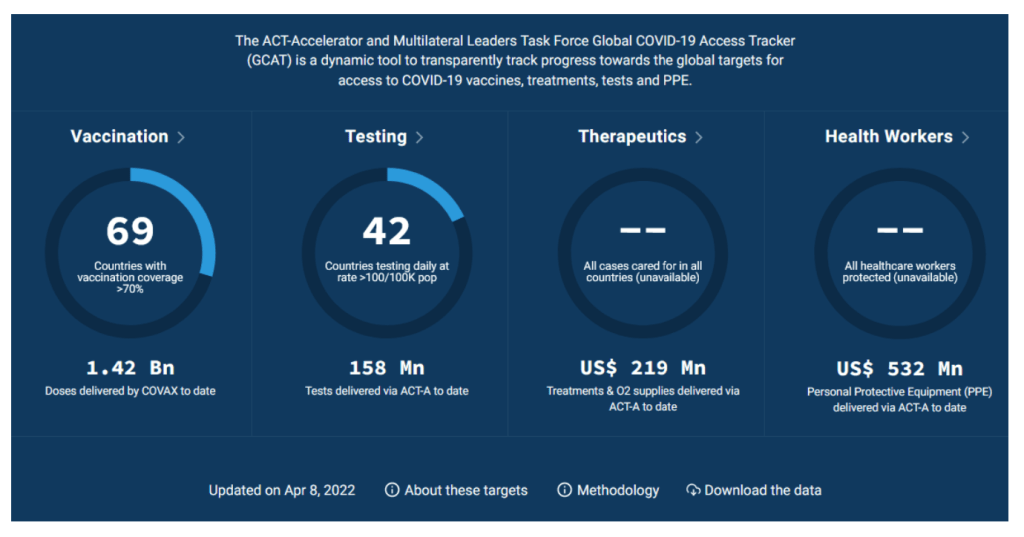
Duke – Launch and Scale Speedometer
The Race for Global COVID-19 Vaccine Equity
A flurry of nearly 200 COVID-19 vaccine candidates are moving forward through the development and clinical trials processes at unprecedented speed; more than ten candidates are already in Phase 3 large-scale trials and several have received emergency or limited authorization. Our team has aggregated and analyzed publicly available data to track the flow of procurement and manufacturing and better understand global equity challenges. We developed a data framework of relevant variables and conducted desk research of publicly available information to identify COVID vaccine candidates and status, deals and ongoing negotiations for procurement and manufacturing, COVID burden by country, and allocation and distribution plans. We have also conducted interviews with public officials in key countries to better understand the context and challenges facing vaccine allocation and distribution
[accessed 24 July 2021]
See our COVID Vaccine Purchases research
See our COVID Vaccine Manufacturing research
See our COVID Vaccine Donations & Exports research

COVID Vaccines – OCHA:: HDX
COVID-19 Data Explorer: Global Humanitarian Operations
COVID-19 Vaccine Roll-out
09 Apr 2022 | COVAX (WHO,GAVI,CEPI), UNDESA, Press Reports | DATA
Global COVID-19 Figures: 494M total confirmed cases; 6.2M total confirmed deaths
Global vaccines administered: 11.4B
Number of Countries: 28
COVAX Allocations Round 4-9 (Number of Doses): 170M
COVAX Delivered (Number of Doses): 280M
Other Delivered (Number of Doses): 260M
Total Delivered (Number of Doses): 540M
Total Administered (Number of Doses): 390M
Multilateral Leaders Task Force on COVID-19 [IMF, World Bank Group, WHO, WTO]
https://data.covid19taskforce.com/data
A global effort to help developing countries access and deliver COVID-19 vaccines, testing, and therapeutics, as they work to end the pandemic and boost economic recovery.
The International Monetary Fund, World Bank Group, World Health Organization and World Trade Organization have joined forces to accelerate access to COVID-19 vaccines, therapeutics and diagnostics by leveraging multilateral finance and trade solutions, particularly in low- and middle-income countries.
Website accessed 09 Apr 2022: https://data.covid19taskforce.com/data The global view below is complemented by country-specific dashboards here.

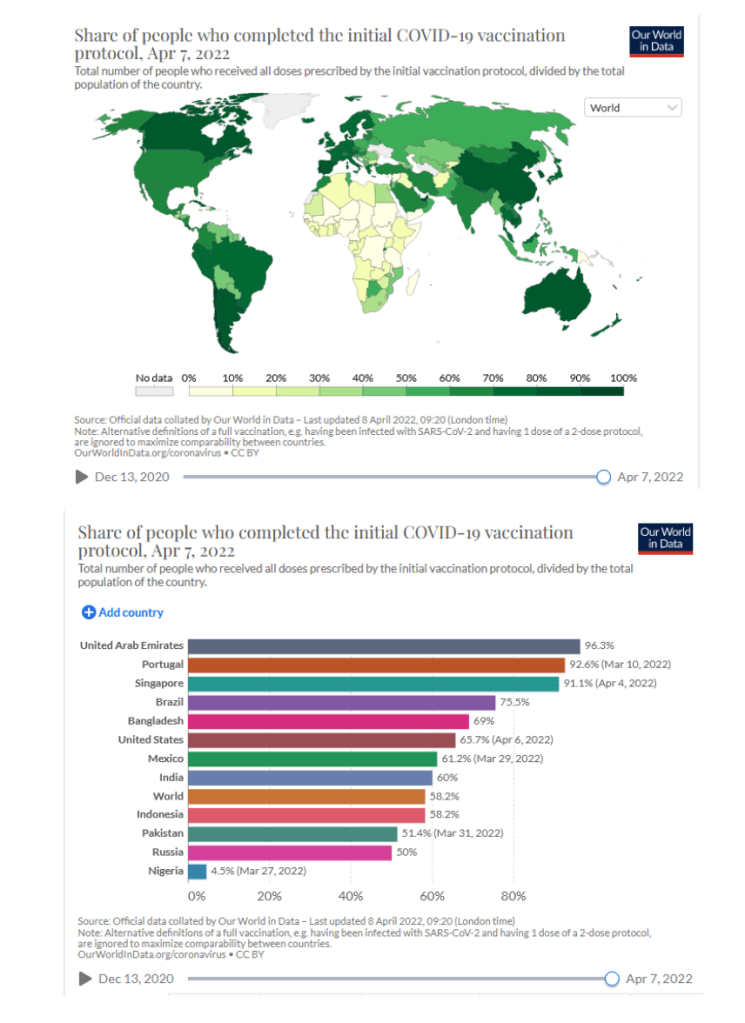
Our World in Data
Coronavirus (COVID-19) Vaccinations [Accessed 09 Apr 2022]
64.7% of the world population has received at least one dose of a COVID-19 vaccine.
11.37 billion doses have been administered globally, and 13.32 million are now administered each day.
Only 14.8% of people in low-income countries have received at least one dose.
U.S.: COVID-19 Vaccines – Announcements/Regulatory Actions/Deployment
HHS
News
Remarks by HHS Secretary Becerra during a joint press conference on World Health Day with the World Health Organization Director-General Dr. Tedros Adhanom Ghebreyesus
April 7, 2022 | Speech
Remarks by Secretary Xavier Becerra at White House COVID-19 Briefing on New Federal Plan to Accelerate Support for People Experiencing Long-Term Effects of COVID-19
April 5, 2022 | Speech
BARDA – U.S. Department of HHS [to 09 Apr 2022]
https://aspr.hhs.gov/newsroom/Pages/NewsRoomHome.aspx
News
No new digest content identified.
::::::
FDA
Press Announcements
No new digest content identified.
Regulatory Actions
Emergency Use Authorization for Vaccines to Prevent COVID-19 Guidance for Industry – March 2022
Final – March 31, 2022
Docket Number: FDA-2020-D-1137
Issued by: Center for Biologics Evaluation and Research
PDF: https://www.fda.gov/media/142749/download
FDA plays a critical role in protecting the United States (U.S.) from threats such as emerging infectious diseases, including the Coronavirus Disease 2019 (COVID-19) pandemic. FDA is committed to providing timely guidance to support response efforts to this pandemic.
FDA is issuing this guidance to provide sponsors of requests for Emergency Use Authorization (EUA) for COVID-19 vaccines with recommendations regarding the data and information needed to support the issuance of an EUA under section 564 of the FD&C Act (21 U.S.C. 360bbb-3) for an investigational vaccine to prevent COVID-19 for the duration of the COVID-19 public health emergency. This document supersedes the guidance of the same title issued in May 2021 (which superseded the guidance of the same title issued October 2020 and reissued February 2021).
Vaccines and Related Biological Products Advisory Committee– FDA
https://www.fda.gov/advisory-committees/blood-vaccines-and-other-biologics/vaccines-and-related-biological-products-advisory-committee
Calendar
Vaccines and Related Biological Products Advisory Committee Meeting April 6, 2022 Announcement – 04/06/2022
The committee met in open session to discuss considerations for use of COVID-19 vaccine booster doses and the process for COVID-19 vaccine strain selection to address current and emerging variants.
[No meeting materials posted as of 09 Apr 2022]
::::::
White House [U.S.] [to 09 Apr 2022]
Briefing Room – Selected Major COVID Announcements
Statement by White House COVID-19 Response Coordinator Jeff Zients on American Lives Saved by the COVID-19 Vaccination Program
April 08, 2022 • Statements and Releases
A new study out today from the Commonwealth Fund shows that President Biden’s relentless efforts to get Americans vaccinated saved millions of American lives. Our vaccination campaign saved 2.2 million American lives, prevented 17 million hospitalizations, prevented 66 million COVID-19 cases, and avoided $900 billion in health care costs….
Statement from President Joe Biden for World Health Day 2022
April 07, 2022 • Statements and Releases
FACT SHEET: The Biden Administration’s Commitment to Global Health in the FY 2023 President’s Budget
April 07, 2022 • Statements and Releases
Press Briefing by White House COVID-19 Response Team and HHS Public Health Officials | April 5, 2022
April 05, 2022 • Press Briefings
Statement by Press Secretary Jen Psaki on COVID-19 Funding Proposal
April 05, 2022 • Statements and Releases
Memorandum on Addressing the Long-Term Effects of COVID-19
April 05, 2022 • Presidential Actions
FACT SHEET: The Biden Administration Accelerates Whole-of-Government Effort to Prevent, Detect, and Treat Long COVID
April 05, 2022 • Statements and Releases
U.S. Department of State [to 09 Apr 2022]
https://www.state.gov/coronavirus/releases/
Press Statement – COVID Context
No new digest content identified.
USAID [to 09 Apr 2022]
https://www.usaid.gov/news-information/press-releases
Selected Press Releases, Statements, Announcements
On the Urgent Need for Additional Global COVID-19 Resources
April 4, 2022
Statement by Administrator Samantha Power
Throughout the COVID-19 pandemic, Congress has consistently come together in an inspiring show of bipartisan support to fund our response. The $10 billion agreement announced this afternoon, while helping to fund our immediate domestic needs like treatments and tests, unfortunately leaves out the resources we need to power our global COVID response—an effort that is critical to preventing the emergence of new deadly variants and moving the world beyond this pandemic.
Europe: COVID-19 Vaccines – Announcements/Regulatory Actions/Deployment
European Medicines Agency
News & Press Releases
Press Releases
News: ECDC and EMA issue advice on fourth doses of mRNA COVID-19 vaccines (new)
Last updated: 06/04/2022
The European Centre for Disease Prevention and Control (ECDC) and EMA’s COVID-19 task force (ETF) have concluded that it is too early to consider using a fourth dose of mRNA COVID-19 vaccines (Pfizer’s Comirnaty and Moderna’s Spikevax) in the general population.
However, both agencies agreed that a fourth dose (or second booster) can be given to adults 80 years of age and above after reviewing data on the higher risk of severe COVID-19 in this age group and the protection provided by a fourth dose.
ECDC and EMA also noted that there is currently no clear evidence in the EU that vaccine protection against severe disease is waning substantially in adults with normal immune systems aged 60 to 79 years and thus no clear evidence to support the immediate use of a fourth dose.
Authorities will continue to monitor data to determine if there is an increasing risk of severe illness among those who are vaccinated…
::::::
European Centre for Disease Prevention and Control
https://www.ecdc.europa.eu/en
Latest Updates [Selected]
[No new digest content identified]
::::::
Accessed 09 Apr 2022
https://vaccinetracker.ecdc.europa.eu/public/extensions/COVID-19/vaccine-tracker.html#uptake-tab

::::::
European Commission
https://ec.europa.eu/commission/presscorner/home/en
Latest [Selected]
Press release 9 April 2022
Stand Up For Ukraine: 9.1 billion euros pledged in support of internally displaced and refugees
The “Stand Up for Ukraine” global pledging event and campaign has raised 9.1 billion euros for people fleeing the Russian invasion, inside Ukraine and abroad, including €1 billion from the European Commission.
Press release 8 April 2022
Ukraine: EU agrees fifth package of restrictive measures against Russia
The European Commission welcomes today’s agreement by the Council to adopt a fifth package of restrictive measures against Putin’s regime in response to its brutal aggression against Ukraine and its people.
Press release 6 April 2022
Food crisis: the EU takes action to support Africa’s Sahel and Lake Chad regions
With the aggravation of the food security and nutrition due to Russia’s invasion of Ukraine, the EU is today reinforcing its political and financial commitment to partner countries in Africa. A total of €554 million in 2022 will be targeted at increasing food security in Sahel and Lake Chad.
Africa: COVID-19 – Announcements/Regulatory Actions/Deployment
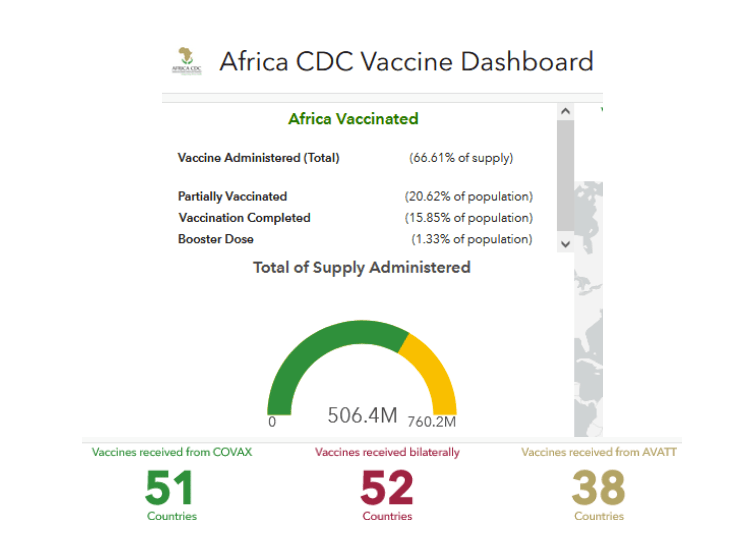
https://africacdc.org/covid-19-vaccination/