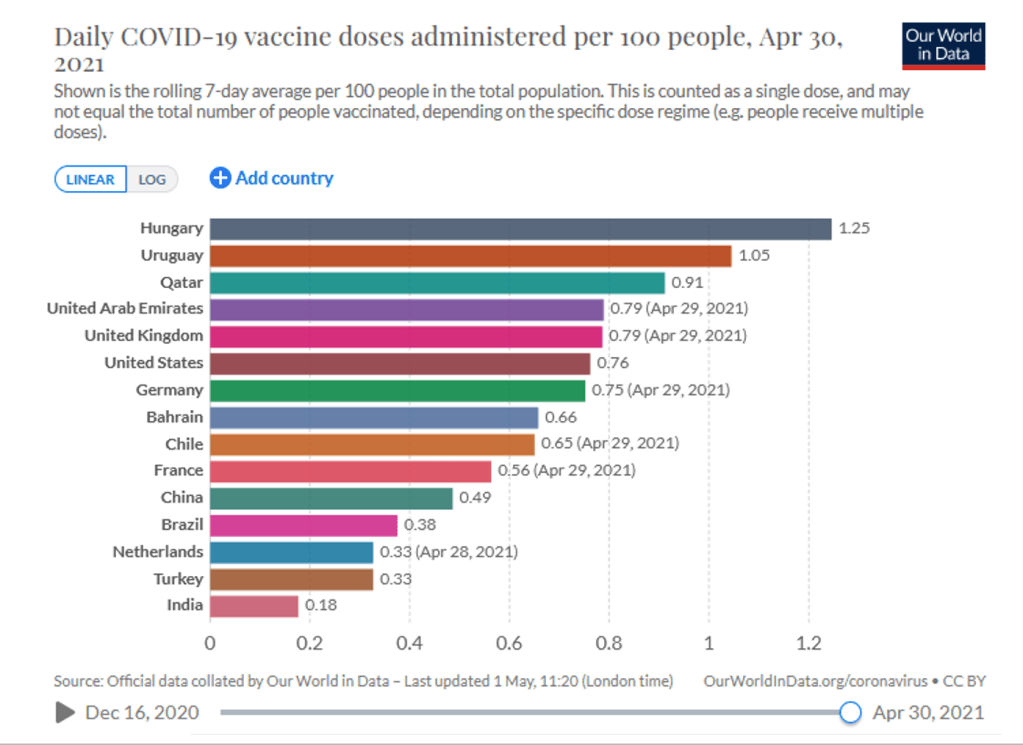
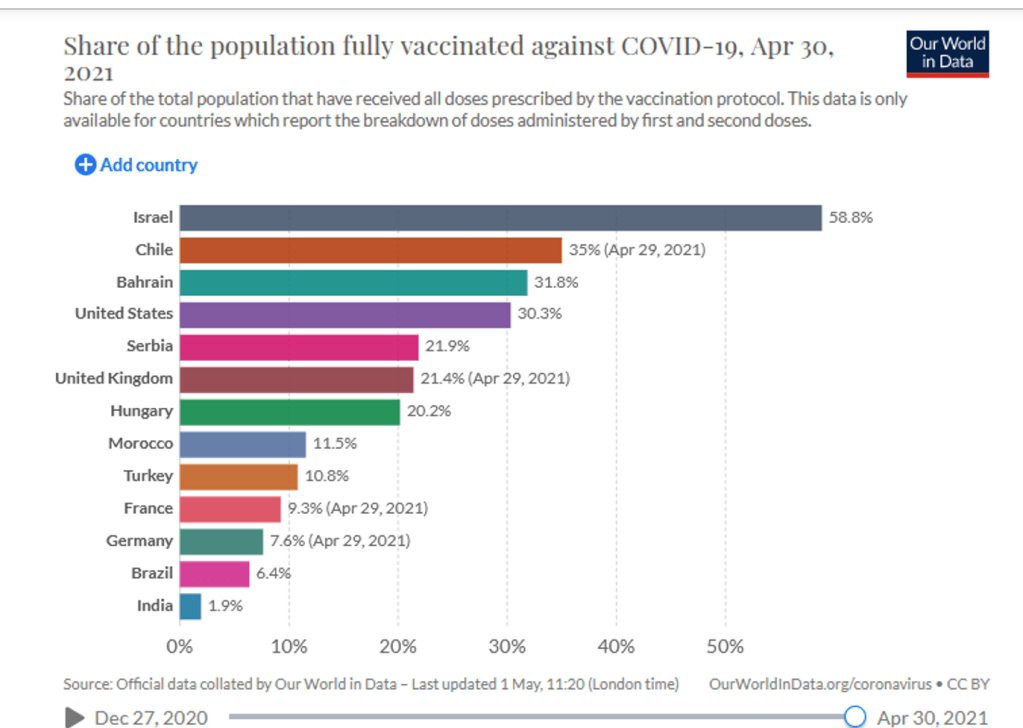
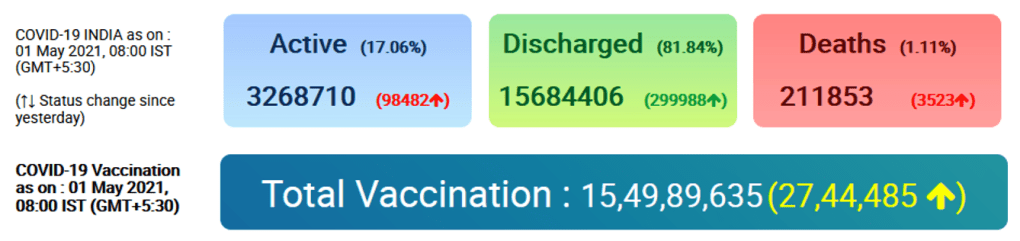
Organization Announcements
Editor’s Note:
Careful readers will note that the number and range of organizations now monitored in our Announcements section below has grown as the impacts of the pandemic have spread across global economies, supply chains and programmatic activity of multilateral agencies and INGOs.
Paul G. Allen Frontiers Group [to 1 May 2021]
https://alleninstitute.org/what-we-do/frontiers-group/news-press/
News
[Website not responding at inquiry]
BARDA – U.S. Department of HHS [to 1 May 2021]
https://www.phe.gov/about/barda/Pages/default.aspx
News
No new digest content identified.
BMGF – Gates Foundation [to 1 May 2021]
https://www.gatesfoundation.org/ideas/media-center
Press Releases and Statements
No new digest content identified.
Bill & Melinda Gates Medical Research Institute [to 1 May 2021]
https://www.gatesmri.org/
The Bill & Melinda Gates Medical Research Institute is a non-profit biotech organization. Our mission is to develop products to fight malaria, tuberculosis, and diarrheal diseases—three major causes of mortality, poverty, and inequality in developing countries. The world has unprecedented scientific tools at its disposal; now is the time to use them to save the lives of the world’s poorest people
No new digest content identified.
CARB-X [to 1 May 2021]
https://carb-x.org/
News
04.27.2021 |
CARB-X is funding UK-biotech GenomeKey to develop a rapid diagnostic for sepsis, a leading cause of death in hospitals
CARB-X is awarding GenomeKey (Genomics Labs Ltd.) in Bristol, UK, up to $3.0 million in non-dilutive funding to develop an innovative rapid diagnostic for sepsis. GenomeKey is eligible for up to $6.5 million in additional awards if the project achieves certain milestones, subject to available funds.
Center for Vaccine Ethics and Policy – GE2P2 Global Foundation [to 1 May 2021]
https://centerforvaccineethicsandpolicy.net/
News/Analysis/Statements
:: Past weekly editions of Vaccines and Global Health: The Week in Review are available here.
:: [NEW] Webinar Recording – Posting of Informed Consent Content on Clinical Trials Registries Center for Informed Consent Integrity Webinar Series – 21 April 2021
CEPI – Coalition for Epidemic Preparedness Innovations [to 1 May 2021]
http://cepi.net/
Latest News
UK support a vital first step in turning tide against future pandemics
The UK Government will host CEPI’s replenishment summit in 2022. This event will be the crucial moment for the world to take action to support our US$3.5 billion plan.
Blog 30 Apr 2021
UK to host global summit with CEPI to speed up new vaccine development
The UK will host a fundraising summit in 2022 to support the work of CEPI.
COVID-19 30 Apr 2021
DARPA – Defense Advanced Research Projects Agency [to 1 May 2021
https://www.darpa.mil/news
News
No new digest content identified.
Duke Global Health Innovation Center [to 1 May 2021]
https://dukeghic.org/
WEEKLY COVID VACCINE RESEARCH UPDATE
Last dated update: FRIDAY, April 16, 2021
EDCTP [to 1 May 2021]
http://www.edctp.org/
The European & Developing Countries Clinical Trials Partnership (EDCTP) aims to accelerate the development of new or improved drugs, vaccines, microbicides and diagnostics against HIV/AIDS, tuberculosis and malaria as well as other poverty-related and neglected infectious diseases in sub-Saharan Africa, with a focus on phase II and III clinical trials
25 April 2021
World Malaria Day 2021
On World Malaria Day 2021, EDCTP joins all partners in the ‘Zero Malaria – Draw the Line Against Malaria’ campaign towards the goal of malaria…
Emory Vaccine Center [to 1 May 2021]
http://www.vaccines.emory.edu/
Vaccine Center News
No new digest content identified.
European Vaccine Initiative [to 1 May 2021]
http://www.euvaccine.eu/
Latest News
European Immunization Week 2021
‘Vaccines bring us closer’ across the world, organisations and generations’
FDA [to 1 May 2021]
https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/default.htm
Press Announcements /Selected Details
April 30, 2021 – Coronavirus (COVID-19) Update: April 30, 2021
April 28, 2021 – FDA Takes Action For Failure to Submit Required Clinical Trial Results Information to ClinicalTrials.Gov
April 27, 2021 – Coronavirus (COVID-19) Update: April 27, 2021
:: On April 23, the FDA and Centers for Disease Control and Prevention (CDC) issued a press release lifting the recommended pause on the use of Janssen (Johnson & Johnson) COVID-19 Vaccine following a thorough safety review. The FDA added and updated some questions about resuming the use of this vaccine to the Janssen COVID-19 Frequently Asked Questions webpage
Fondation Merieux [to 1 May 2021]
http://www.fondation-merieux.org/
News, Events
No new digest content identified.
Gavi [to 1 May 2021]
https://www.gavi.org/
News Releases
26 April 2021
Immunization services begin slow recovery from COVID-19 disruptions, though millions of children remain at risk from deadly diseases
[See COVAX above for detail]
GHIT Fund [to 1 May 2021]
https://www.ghitfund.org/newsroom/press
GHIT was set up in 212 with the aim of developing new tools to tackle infectious diseases that
No new digest content identified.
Global Fund [to 1 May 2021]
https://www.theglobalfund.org/en/news/
News & Stories
India’s devastating COVID-19 crisis
30 April 2021
…As part of the Global Fund’s COVID-19 Response Mechanism 2021, we have made available an initial base allocation of US$75 million to support India’s response to COVID-19. We are working closely with the Government of India and national partners to help them access the new emergency funding and identify solutions to the most critical needs.
The Global Fund is also working with international partners to support India. In February 2021, the COVID-19 Oxygen Emergency Taskforce was launched by Unitaid and the Wellcome Trust as co-leaders of the Access to COVID-19 Tools Accelerator (ACT-Accelerator) Therapeutics Pillar, to respond to the surging oxygen emergency in low- and middle-income countries. The taskforce brings together key organizations including Unitaid, the Wellcome Trust, the Global Fund, WHO, Unicef, World Bank, the Clinton Health Access Initiative (CHAI), PATH, the Every Breath Counts coalition and Save the Children…
Global Research Collaboration for Infectious Disease Preparedness [GloPID-R] [to 1 May 2021]
https://www.glopid-r.org/news/
News
No new digest content identified.
Hilleman Laboratories [to 1 May 2021]
http://www.hillemanlabs.org/
Website not responding at inquiry
Human Vaccines Project [to 1 May 2021]
http://www.humanvaccinesproject.org/media/press-releases/
No new digest content identified.
[Website not fully loading at inquiry]
IAVI [to 1 May 2021]
https://www.iavi.org/newsroom
PRESS RELEASES/FEATURES
No new digest content identified.
International Coalition of Medicines Regulatory Authorities [ICMRA]
http://www.icmra.info/drupal/en/news
Selected Statements, Press Releases, Research
No new digest content identified.
ICRC [to 1 May 2021]
https://www.icrc.org/en/whats-new
Selected News Releases, Statements, Reports
Legislative Checklist: Protecting Health Care from Violence
The document presents a list of questions (a checklist or compatibility study) that cover some of the main challenges related to the protection of health care during armed conflict and other emergencies.
28-04-2021 | Publication
Without urgent action to protect essential services in conflict zones we face vast humanitarian disaster
Speech given by Mr Peter Maurer, President of the International Committee of the Red Cross, to the UNSC Open Debate on the Protection of Objects Indispensable to the survival of the civilian population.
27-04-2021 | Statement
International Generic and Biosimilar Medicines Association [IGBA]
https://www.igbamedicines.org/
News
No new digest content identified.
IFFIm
http://www.iffim.org/
Press Releases/Announcements
No new digest content identified.
IFRC [to 1 May 2021]
http://media.ifrc.org/ifrc/news/press-releases/
Selected Press Releases, Announcements
Americas, Argentina, Brazil, Chile, Costa Rica, Paraguay, Peru, Uruguay
IFRC calls for an urgent scale-up of vaccinations, health interventions and economic support as the pandemic continues to surge in the Americas
As the Americas region continues to see a surge in COVID-19 cases and deaths, the International Federation of Red Cross and Red Crescent Societies (IFRC) is calling for an urgent scaling up of public health interventions, vaccinations and economic support for the most vulnerable. With almost half of the cases and deaths from COVID-19 in the world, the continent continues to bear the brunt of this monumental crisis on various fronts.
28 April 2021
India
IFRC urges unity to vaccinate the world against COVID-19 as countries unite to help India
Kuala Lumpur/Delhi/Geneva, 28 April – The terrible toll of death and illness unfolding in India reinforces that this pandemic is far from over. The International Federation of Red Cross and Red Crescent Societies (IFRC) urges the whole world to unify a …
28 April 2021
Philippines
Philippines: Red Cross eases burden on hospitals under siege as COVID-19 cases hit 1 million
Kuala Lumpur/Manila, 26 April – Record COVID-19 surges across the Philippines have placed hospitals under siege as total Coronavirus infections pass 1 million. Red Cross is urgently setting up field hospitals, quarantine facilities and scaling up exist …
26 April 2021
Institut Pasteur [to 1 May 2021]
https://www.pasteur.fr/en/press-area
Press release
No new digest content identified.
IRC International Rescue Committee [to 1 May 2021]
http://www.rescue.org/press-release-index
Media highlights [Selected]
Press Release
NE Syria: As COVID surges, hospitals face acute O2 shortage and testing is in jeopardy; IRC makes urgent call for more funding and support to boost COVID response
April 27, 2021
IVAC [to 1 May 2021]
https://www.jhsph.edu/research/centers-and-institutes/ivac/index.html
Updates; Events
No new digest content identified.
IVI [to 1 May 2021]
http://www.ivi.int/
Selected IVI News, Announcements, Events
No new digest content identified.
JEE Alliance [to 1 May 2021]
https://www.jeealliance.org/
Selected News and Events
No new digest content identified.
Johns Hopkins Center for Health Security [to 1 May 2021]
https://www.centerforhealthsecurity.org/news/center-news/
Center News
No new digest content identified.
MSF/Médecins Sans Frontières [to 1 May 2021]
http://www.msf.org/
Latest [Selected Announcements
Coronavirus COVID-19 pandemic
Responding to COVID-19: Global Accountability Report 3 – September to December 2020
Report = 29 Apr 2021
… From March to December 2020, Médecins Sans Frontières (MSF) responded to the pandemic through our existing programmes and dedicated COVID-19 interventions in more than 300 projects in 70 countries. Our global COVID-19 activities focused on delivering medical care and other assistance to vulnerable people at risk of being left behind, including remote communities, people on the move, homeless people, and elderly people living in long-term care facilities…
National Academy of Medicine – USA [to 1 May 2021]
https://nam.edu/programs/
Selected News/Programs
Upcoming Events:
Collaborating with Communities about COVID-19, Climate, and Community Concerns: A Roundtable Discussion
May 4, 2021 1:00PM – 2:30PM (ET)
COVID-19 Vaccines: Building Confidence and Explaining Efficacy, Webinar
May 4, 2021 3:00PM – 4:00PM (ET)
National Vaccine Program Office – U.S. HHS [to 1 May 2021]
https://www.hhs.gov/vaccines/about/index.html
Upcoming Meetings/Latest Updates
No new digest content identified.
NIH [to 1 May 2021]
http://www.nih.gov/news-events/news-releases
News Releases
NIH to invest $29 million to address COVID-19 disparities
April 29, 2021 — Effort will foster community-engagement research in communities which have been hit hardest by the pandemic.
UN OCHA Office for the Coordination of Humanitarian Affairs [to 1 May 2021]
https://www.unocha.org/
Press Releases
No new digest content identified.
PATH [to 1 May 2021]
https://www.path.org/media-center/
Press Releases
PATH welcomes Samantha Power as new USAID administrator
April 28, 2021 by PATH
Sabin Vaccine Institute [to 1 May 2021]
http://www.sabin.org/updates/pressreleases
Statements and Press Releases
No new digest content identified.
UNAIDS [to 1 May 2021]
http://www.unaids.org/en
Selected Press Releases/Reports/Statements
30 April 2021
Joint mission supports the response to HIV in Gboklè/Nawa/San Pedro, Côte d’Ivoire
26 April 2021
High rates of hepatitis C and HIV coinfection among key populations
26 April 2021
Interactive multistakeholder hearing takes place ahead of United Nations High-Level Meeting on AIDS
UNDP United Nations Development Programme [to 1 May 2021]
http://www.undp.org/content/undp/en/home/presscenter.html
Latest from News Centre
Pandemic and political crisis could result in half of Myanmar’s population living in poverty by 2022, UNDP says
The combined effects of the two crises could put over a decade of progress on poverty reduction in the country at risk.
Posted on April 30, 2021
UNESCO [to 1 May 2021]
http://en.unesco.org/news
Selected Latest News
No new digest content identified.
UNHCR Office of the United Nations High Commissioner for Refugees [to 1 May 2021]
http://www.unhcr.org/en-us/media-centre.htmlS
Selected News Releases, Announcements
No new digest content identified.
UNICEF [to 1 May 2021]
https://www.unicef.org/media/press-releases
Selected Press Releases, Statements
News note
04/29/2021
UNICEF sends 3,000 oxygen concentrators and other critical supplies to India as country battles deadly COVID-19 surge
Press release
04/28/2021
Vaccination Demand Observatory launched to strengthen local communication programmes to address vaccine misinformation
UNICEF, Yale Institute for Global Health, and Public Good Projects team up to create the Vaccination Demand Observatory to equip country teams with tools to counter misinformation and mistrust related to all vaccines
…The Vaccine Acceptance Interventions Lab (VAIL) will draw upon behavioural and social research and insights from social listening to develop engaging, relevant content to fill information gaps. VAIL also will develop “inoculation” messages to vaccinate people against vaccine misinformation. The content and programs will be rapid field tested for tone, format and behavior change impact before being implemented…
Statement
04/26/2021
UNICEF Executive Director Henrietta Fore’s remarks at the special press briefing with WHO and Gavi on the impact of COVID-19 on immunization
As prepared for delivery
[See Milestones above for detail]
Press release
04/26/2021
Immunization services begin slow recovery from COVID-19 disruptions, though millions of children remain at risk from deadly diseases – WHO, UNICEF, Gavi
Ambitious new global strategy aims to save over 50 million lives through vaccination
[See Milestones above for detail]
Unitaid [to 1 May 2021]
https://unitaid.org/
Featured News
28 April 2021
Unitaid and Wellcome statement regarding MSD’s global access plan for molnupiravir, a potential treatment for COVID-19
Geneva – ACT-Accelerator Therapeutics co-leads Wellcome and Unitaid welcome MSD’s commitment to voluntary licensing agreements with five generic manufacturers to serve the needs of India and 100 other low- and middle-income countries (LMICs). This is an important first step to accelerate and expand global access for molnupiravir, should upcoming clinical data prove it safe and effective.
Industry plays a central role in ensuring a rapid and effective COVID-19 response. Their commitment to the development of promising therapeutics for COVID-19 is critical in the race to end the pandemic and in meeting the continued need for treatments for COVID-19.
We also welcome MSD’s engagement with the Unitaid-supported Medicines Patent Pool (MPP) to further broaden access to molnupiravir. In anticipation of a potential approval in the months to come, robust plans for affordable access in all low- and middle-income countries are required, including middle-income countries in Africa, Asia and Latin America, given the trajectory of the pandemic.
28 April 2021
Access to HIV self-tests significantly expanded and costs halved thanks to Unitaid agreement
:: Self-testing vital tool to help people know HIV status – but market has been dominated by single oral-based self-test
:: New Unitaid-led agreement with Viatris (through its subsidiary Mylan) reduces price of blood-based HIV self-test by 50%, significantly expanding market and giving countries more options
:: New test will be available for less than $2 in 135 countries
:: Self tests key factor in achieving SDG target of 90% of people infected with HIV knowing their status
27 April 2021
ANTICOV: largest clinical trial in Africa for people with mild COVID-19 to test new drug combination
Need for effective COVID-19 treatments remains particularly acute, especially in sub-Saharan Africa where access to vaccines is still extremely limited
Geneva – The ANTICOV clinical trial, conducted in 13 African countries, has started the recruitment of participants to test a new drug combination, nitazoxanide + ciclesonide, to treat people with mild-to-moderate COVID-19 before their cases become severe.
The ANTICOV study is being carried out by a consortium of 26 partners which include leading African research institutions and international health organizations, coordinated by the non-profit research and development (R&D) organization Drugs for Neglected Diseases initiative (DNDi). It is the largest trial in Africa to identify early COVID-19 treatments that can prevent progression to severe disease and potentially limit transmission.
“In many African countries our worst fears are being realised, as already-strained intensive care units are beginning to fill up with COVID-19 patients,” said Dr John Nkengasong, Director of the Africa Centres for Disease Control and Prevention (Africa CDC). “Yet the number of vaccine doses that are reaching the African continent is too limited. The rapid spread of new variants also threatens to reduce the efficacy of existing vaccines, which is another major cause for concern. We need urgently to identify affordable and easy-to-administer treatments that can prevent the evolution to a severe form of the disease and slow the rate of infection.”…
Vaccination Acceptance Research Network (VARN) [to 1 May 2021]
https://vaccineacceptance.org/news.html#header1-2r
Announcements
No new digest content identified.
Vaccine Confidence Project [to 1 May 2021]
http://www.vaccineconfidence.org/
News, Research and Reports
Coronavirus global impact
Launched April 2, 2020 and recurring every 3 days, Premise Data is utilizing its global network of Contributors to assess economic, social, and health sentiment surrounding the coronavirus (COVID-19).
Vaccine Education Center – Children’s Hospital of Philadelphia [to 1 May 2021]
http://www.chop.edu/centers-programs/vaccine-education-center
News
No new digest content identified.
Wellcome Trust [to 1 May 2021]
https://wellcome.ac.uk/news
News and reports
Explainer
Why we need to share vaccine doses now and why COVAX is the right way to do it
26 April 2021
We need urgent international action to achieve more equitable global access to Covid-19 vaccines. Countries that have ordered more vaccine doses than they need are best placed to take the lead, by sharing doses now, through COVAX.
The Wistar Institute [to 1 May 2021]
https://www.wistar.org/news/press-releases
Press Releases
Apr. 27, 2021
Wistar Expands International Training of the Next Generation of Scientists With the University of Bologna
Second international training program offers mentorship under top cancer research scientists.
WFPHA: World Federation of Public Health Associations [to 1 May 2021]
https://www.wfpha.org/
Latest News
COVID-19 a Springboard for Immunization for All Ages
News Apr 29, 2021
The WFPHA has used World Immunisation Week to call for greater coordination in the vaccination of people of all ages.
World Bank [to 1 May 2021]
http://www.worldbank.org/en/news/all
Selected News, Announcements
World Bank Expands Colombia Amazon Conservation Project with US$18.4 Million Grant
Washington, D.C., April 30, 2021 – The World Bank Board of Directors today approved an additional grant of US$18.4 million from the Global Environment Facility (GEF) for the Forest Conservation…
Date: April 30, 2021 Type: Press Release
Lebanon Financing Facility Approves $55 million Recovery Program Targeting Vulnerable Households and Small Businesses to Respond to the Port of Beirut Explosion
Beirut, April 30, 2021 – The World Bank convened yesterday the first meeting of the Partnership Council for the Lebanon Financing Facility (LFF) to discuss the priorities, proposed funding allocations…
Date: April 29, 2021 Type: Press Release L
Lebanon Financing Facility for Reform, Recovery and Reconstruction (LFF)- Questions and Answers
Following the Port of Beirut explosion on August 4, 2020, the World Bank Group, the European Union and the United Nations developed the Reform, Recovery and Reconstruction Framework (3RF) in order to address…
Date: April 29, 2021 Type: Brief
Living with Debt: How Institutions Can Chart a Path to Recovery in the Middle East and North Africa
Authors: Gatti, Roberta; Lederman, Daniel; Nguyen, Ha M.; Alturki, Sultan Abdulaziz.; Fan, Rachel Yuting; Islam, Asif M.; Rojas, Claudio More than a year after the World Health Organization declared…
Date: April 28, 2021 Type: Brief
Supporting teachers using technology: 400 solutions from 80 countries
The World Bank Group and HundrED teamed up, supported with funding from the Global Partnership for Education (GPE), to launch the Teachers for a Changing World: Transforming Teacher Professional Development…
Date: April 28, 2021 Type: Feature Story
What is learning poverty?
All children should be able to read by age 10. Reading is a gateway for learning as the child progresses through school—and conversely, an inability to read slams that gate shut. Beyond this, when children…
Date: April 28, 2021 Type: Brief
Moldova’s Vaccination System to be Strengthened, with World Bank Support
WASHINGTON, April 27, 2021 – The World Bank has approved Additional Financing for the Moldova COVID-19 Emergency Response Project in the amount of EUR 24.8 million, which will support the Government of…
Date: April 27, 2021 Type: Press Release
World Bank Supports Fair and Effective Deployment of COVID-19 Vaccines in Sri Lanka
WASHINGTON, April 27, 2021—The World Bank today approved $80.5 million additional financing to help Sri Lanka access and distribute COVID-19 vaccines and to strengthen the country’s vaccination system…
Date: April 27, 2021 Type: Press Release
World Customs Organization – WCO [to 1 May 2021]
http://www.wcoomd.org/
Latest News – Selected Items
No new digest content identified.
World Organisation for Animal Health (OIE) [to 1 May 2021]
https://www.oie.int/en/for-the-media/press-releases/2021/
Press Releases
No new digest content identified.
WTO – World Trade Organisation [to 1 May 2021]
http://www.wto.org/english/news_e/news_e.htm
WTO News and Events
No new digest content identified.
::::::
ARM [Alliance for Regenerative Medicine] [to 1 May 2021]
Press Releases – Alliance for Regenerative Medicine (alliancerm.org)
Press Releases
No new digest content identified.
BIO [to 1 May 2021]
https://www.bio.org/press-releases
Press Releases
No new digest content identified.
DCVMN – Developing Country Vaccine Manufacturers Network [to 1 May 2021]
http://www.dcvmn.org/
News; Upcoming events
No new digest content identified.
ICBA – International Council of Biotechnology Associations [to 1 May 2021]
https://internationalbiotech.org/news/
News
No new digest content identified.
IFPMA [to 1 May 2021]
http://www.ifpma.org/resources/news-releases/
Selected Press Releases, Statements, Publications
IFPMA statement at the close of the 3rd Fair Pricing Forum 13 to 22 April
Published on: 23 April 2021
We thank the organizers, World Health Organization and Ministry of Health of Argentina for convening this meeting and for inviting representatives from the innovative biopharmaceutical industry to share our experience and perspectives on how we can work collectively toward improved access to medicines worldwide.
It is with this mindset that we engaged in a constructive and open dialogue, aimed at co-creating solutions to our common objective of bringing innovative medicines to more patients around the world. We remain committed to maintaining the course in reaching the Sustainable Development Goals health targets, despite the coronavirus pandemic. The innovative biopharmaceutical industry is sensitive to the debate about affordability and access. We are making progress through new partnerships in reaching many more patients and supporting health systems for sustainable and longstanding improvements to patient health outcomes. There is still work to be done; and there is no room for complacency.
The coronavirus pandemic has shown how the innovative biopharmaceutical industry has risen to the occasion and to meet societal expectations in developing and scaling up manufacturing of tests, treatments, and vaccines, driven by a strong belief that in the end science will win and help us to overcome the pandemic. There are challenges to overcome in meeting the goal of “no one is safe until everyone is safe”, but we have also seen the power of public-private partnerships, such as Covax, and the critical role of vaccine manufacturers from industrialized as well as developing countries in scaling up production from zero to billions of doses in record time.
As discussed in prior editions of the Fair Pricing Forum, “fair pricing and fair access” should be achieved while ensuring continued support for an innovation ecosystem that allows future discoveries for today and tomorrow’s unmet medical needs. The COVID-19 innovations and roll out that have been achieved for the pandemic as a response to the biggest public health crisis of our age, cannot be assumed to be the norm. The unprecedented actions industry has taken for the coronavirus public health pandemic IS NOT BUSINESS AS USUAL. We have been able to respond in the manner that we have by leaning on the knowledge, know-how and pillars of a functioning innovation eco-system, built on decades of on-going investment.
We want to be better-prepared for future pandemics. To enable this, the fundamentals that have enabled our industry’s readiness to swiftly take on the COVID challenge should not be compromised.
PhRMA [to 1 May 2021]
http://www.phrma.org/
Selected Press Releases, Statements
New PhRMA report shows nearly 90 medicines in development to fight drug-resistant infections, but future pipeline remains challenging
April 29, 2021
New PhRMA report shows nearly 90 medicines in development to fight drug-resistant infections, but future pipeline remains challenging
Blog Post
Patient safety is at the heart of COVID-19 vaccine R&D
April 26, 2021
Patient safety is at the heart of COVID-19 vaccine R&D. Robust clinical research standards, safety monitoring, expert guidance, and genomic surveillance efforts all contribute to a robust safety practice surrounding our vaccine manufacturing and distribution ecosystem.
Blog Post