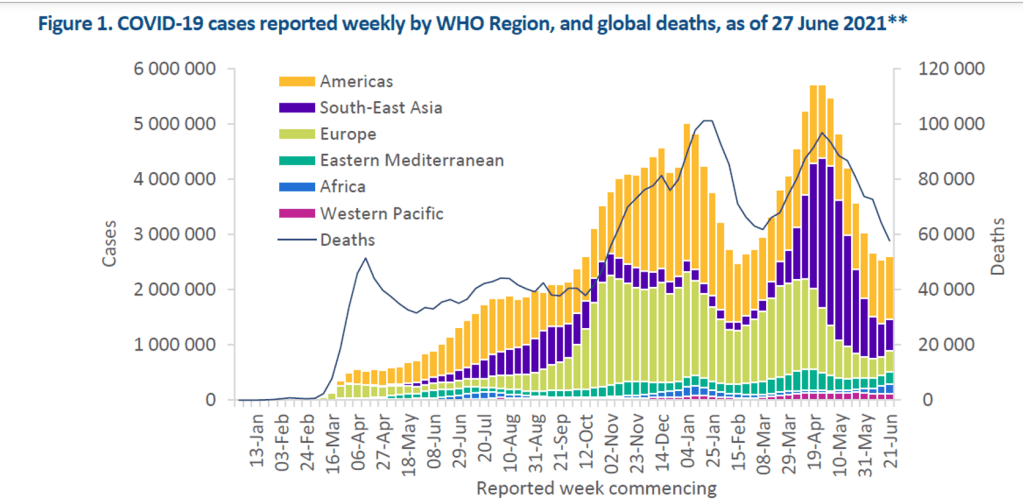
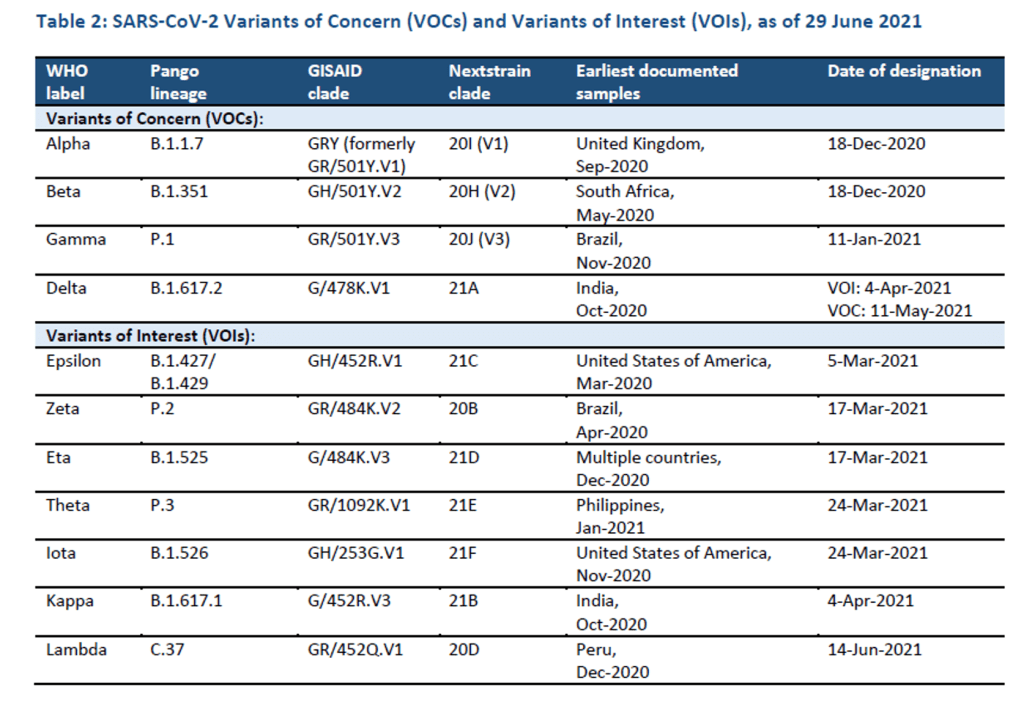
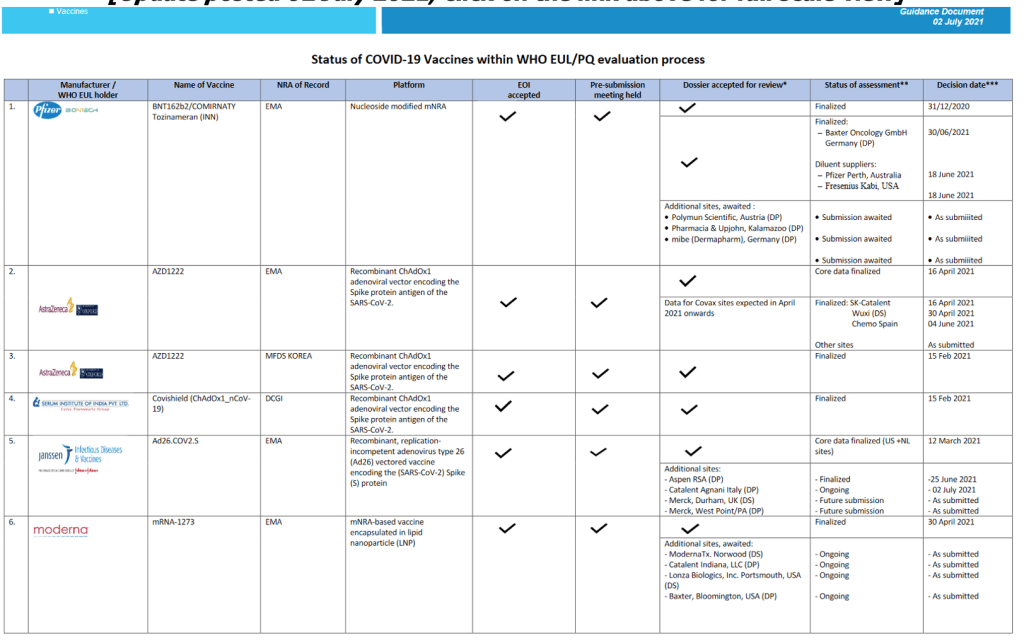
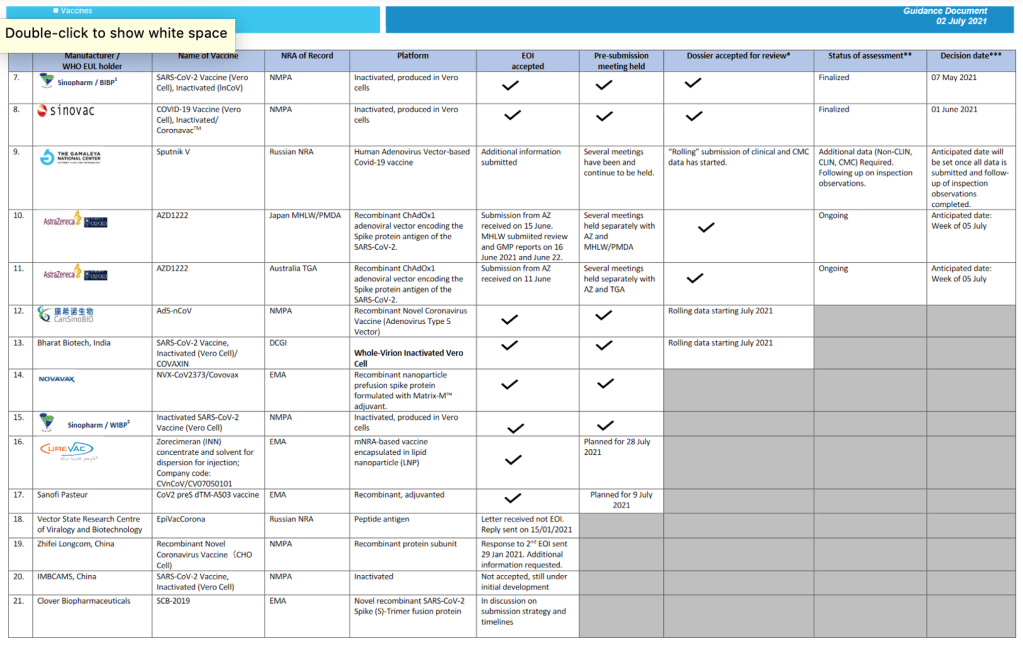
Milestones :: Perspectives :: Research
World Bank Financing for COVID-19 Vaccine Rollout Exceeds $4 Billion for 50 Countries
WASHINGTON, June 30, 2021 – The World Bank announced today that it is providing over $4 billion for the purchase and deployment of COVID-19 vaccines for 51 developing countries, half of which are in Africa. More than half of the financing comes from the International Development Association (IDA), the Bank’s fund for the world’s poorest countries, and is on grant or highly concessional terms. This financing is part of the Bank’s commitment to help low- and middle-income countries acquire and distribute vaccines and strengthen their health systems.
The World Bank reiterated its call to governments, pharmaceutical companies, and organizations involved in vaccine procurement and delivery to help increase transparency and build greater public information regarding vaccine contracts, options and agreements; vaccine financing and delivery agreements; and doses delivered and future delivery plans. It asked those countries anticipating excess vaccine supplies in the coming months to release their surplus doses and options as soon as possible, in a transparent manner, to developing countries with adequate distribution plans in place.
Since the start of the COVID-19 pandemic, the World Bank Group has approved more than $150 billion to fight the health, economic, and social impacts of the pandemic. Since April 2020, the Bank has scaled up its financing by over 50 percent, helping more than 100 countries meet emergency health needs, strengthen pandemic preparedness, while also supporting countries as they protect the poor and jobs, and jump starting a climate-friendly recovery.
“The World Bank is helping developing countries in every region of the world with vaccine purchase and rollout,” said Axel van Trotsenburg, World Bank Managing Director of Operations. “Significant challenges still remain regarding vaccine deployment and hesitancy. We are taking action on all fronts to tackle these challenges, working in solidarity with international and regional partners to expedite doses to as many people as possible and to enhance disease surveillance, preparedness, and response.”
Full details of World Bank vaccine operations are posted on our vaccine operations portal, with regular updates. The $4 billion is supporting COVID-19 vaccination efforts in Afghanistan, Bangladesh, Benin, Cabo Verde, Cambodia, Comoros, the Republic of Congo, Côte d’Ivoire, Democratic Republic of Congo, Ecuador, El Salvador, Eswatini, Ethiopia, The Gambia, Georgia, Ghana, Guinea, Guinea Bissau, Guyana, Honduras, Indonesia, Jordan, Kenya, Kosovo, the Kyrgyz Republic, Lao PDR, Lebanon, Lesotho, Madagascar, Malawi, Moldova, Mongolia, Mozambique, Nepal, Niger, Pakistan, Papua New Guinea, Philippines, Rwanda, São Tomé e Príncipe, Senegal, Sierra Leone, South Sudan, Sri Lanka, Sudan, Tajikistan, Togo, Tunisia, Ukraine, Yemen, and Zambia.
The Bank’s vaccine finance package is designed to be flexible. It can be used by countries to acquire doses through COVAX, the Africa Vaccine Acquisition Task Team (AVATT) or other sources. It also finances vaccine deployment and health system strengthening, such as vaccine cold-chains, training health workers, data and information systems, and communications and outreach campaigns to key stakeholders which are crucial to ensure vaccination acceptance. The Bank has aligned its eligibility criteria for COVID-19 vaccines with the revised eligibility criteria of COVAX and other multilateral partners.
The World Bank is partnering with the African Union and the World Bank-supported Africa Center for Disease Control to support AVATT initiative with resources to allow countries to purchase and deploy vaccines for up to 400 million people across Africa. The Bank is also convening a task force with the IMF, WHO, WTO, and other partners to track, coordinate, and advance delivery of COVID-19 vaccines to developing countries.
The Bank continues to work with governments and partners (UNICEF, the Global Fund, WHO, and GAVI) to assess the readiness of over 140 developing countries to deploy vaccines. Countries have made good progress since the publication of the effort’s first report. Latest findings show that 95 percent of countries have developed national vaccination plans, 79 percent have safety measures in place, and 82 percent have prioritizations of populations to receive the vaccine. However, only 59 percent have developed plans to train the large number of vaccinators needed and less than half have a plan in place to generate public confidence, trust, and demand for COVID-19 vaccines.
::::::
World Bank Vaccine Operations Portal
https://www.worldbank.org/en/who-we-are/news/coronavirus-covid19/world-bank-support-for-country-access-to-covid-19-vaccines
Countries receiving World Bank support for vaccines
As of July 1, 2021
This list of countries, project documents, and procurement notices and contracts at the link above will be updated as data becomes available.