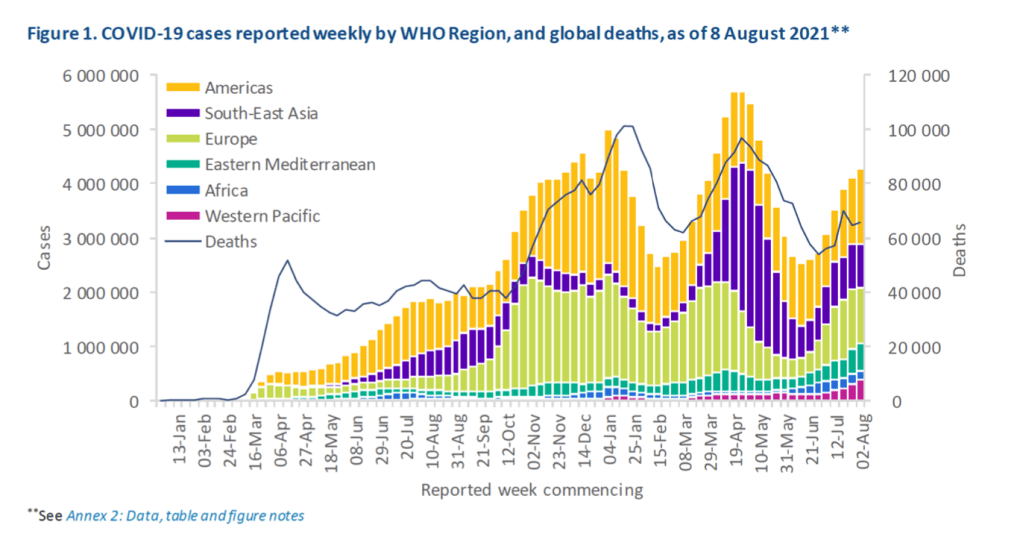
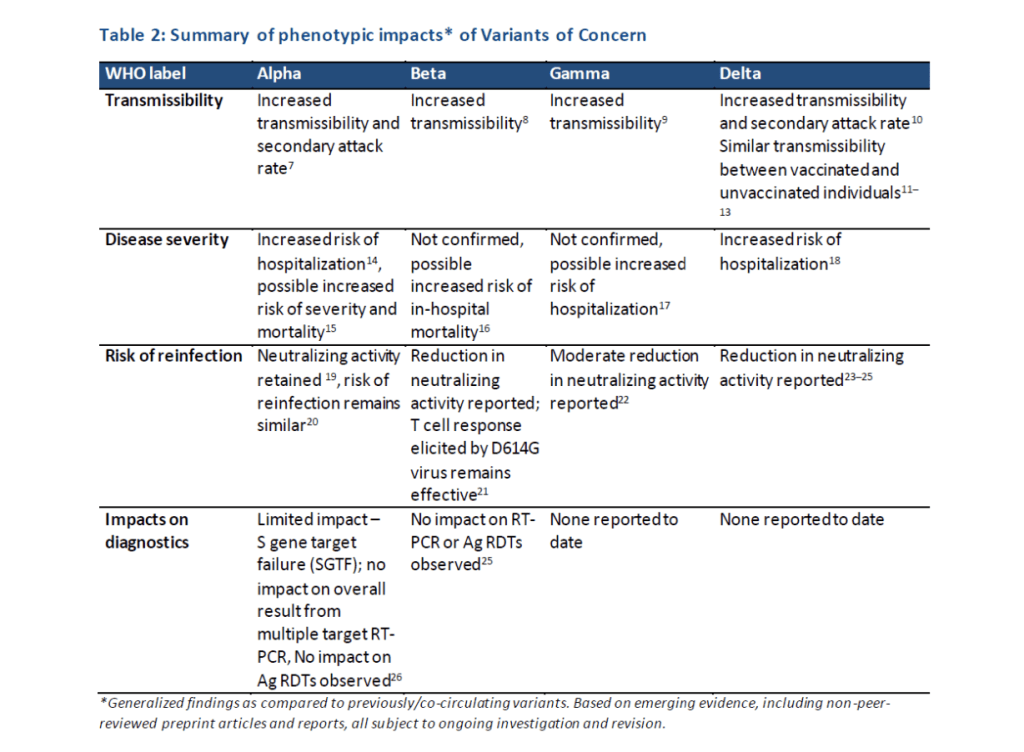
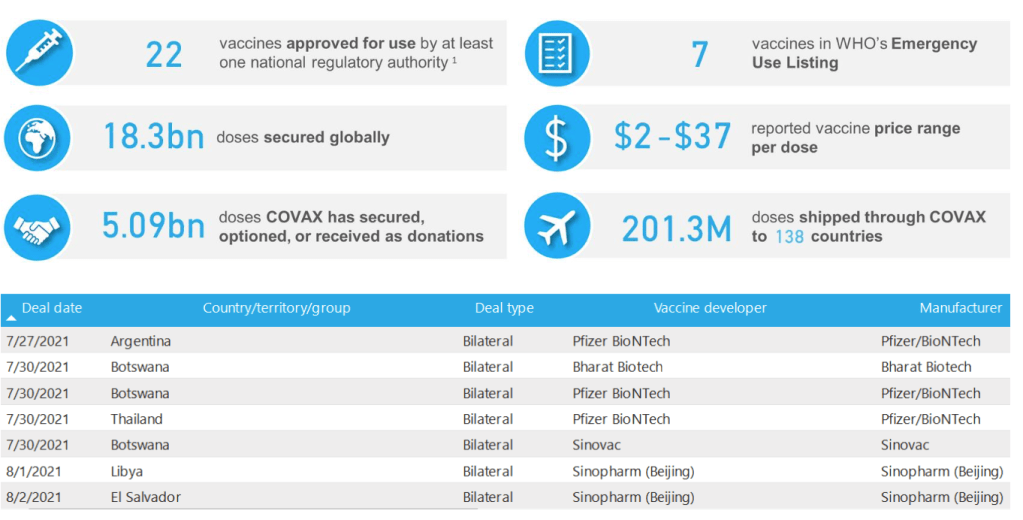
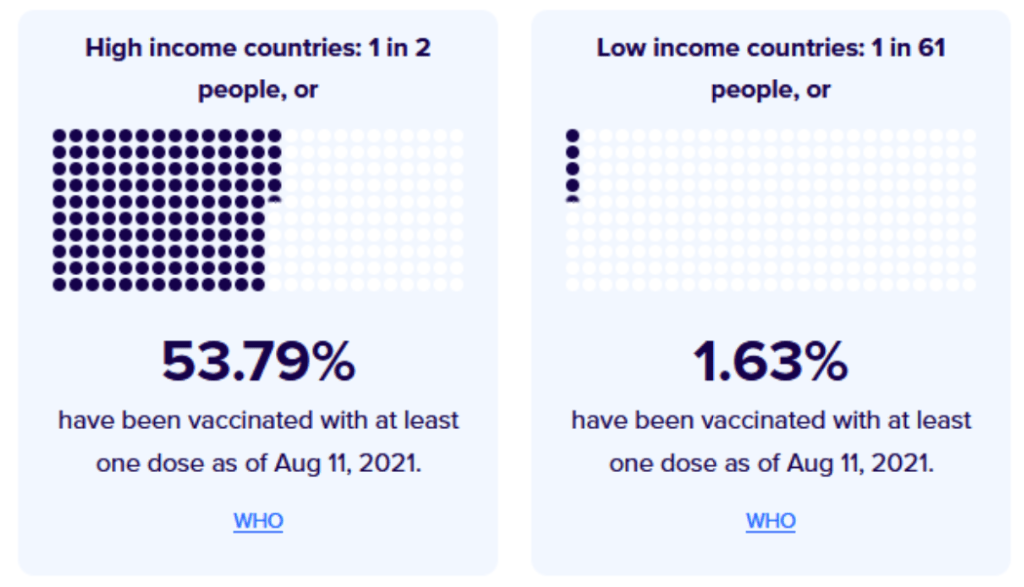
::::::
Organization Announcements
Editor’s Note:
Careful readers will note that the number and range of organizations now monitored in our Announcements section below has grown as the impacts of the pandemic have spread across global economies, supply chains and programmatic activity of multilateral agencies and INGOs.
Paul G. Allen Frontiers Group [to 14 Aug 2021]
https://alleninstitute.org/what-we-do/frontiers-group/news-press/
News
No new digest content identified.
BARDA – U.S. Department of HHS [to 14 Aug 2021]
https://www.phe.gov/about/barda/Pages/default.aspx
News
No new digest content identified.
BMGF – Gates Foundation [to 14 Aug 2021]
https://www.gatesfoundation.org/ideas/media-center
Press Releases and Statements
No new digest content identified.
Bill & Melinda Gates Medical Research Institute [to 14 Aug 2021]
https://www.gatesmri.org/
The Bill & Melinda Gates Medical Research Institute is a non-profit biotech organization. Our mission is to develop products to fight malaria, tuberculosis, and diarrheal diseases—three major causes of mortality, poverty, and inequality in developing countries. The world has unprecedented scientific tools at its disposal; now is the time to use them to save the lives of the world’s poorest people
No new digest content identified.
CARB-X [to 14 Aug 2021]
https://carb-x.org/
News
No new digest content identified.
Center for Vaccine Ethics and Policy – GE2P2 Global Foundation [to 14 Aug 2021]
https://centerforvaccineethicsandpolicy.net/
News/Analysis/Statements
:: Past weekly editions and posting of all segments of Vaccines and Global Health: The Week in Review are available here.
:: [NEW] Informed Consent: A Monthly Review – August 2021 is now posted here
CEPI – Coalition for Epidemic Preparedness Innovations [to 14 Aug 2021]
http://cepi.net/
Latest News
No new digest content identified.
CIOMS – COUNCIL FOR INTERNATIONAL ORGANIZATIONS OF MEDICAL SCIENCES [to 14 Aug 2021]
https://cioms.ch/
News; Publications
No new digest content identified.
DARPA – Defense Advanced Research Projects Agency [to 14 Aug 2021
https://www.darpa.mil/news
News
No new digest content identified.
Duke Global Health Innovation Center [to 14 Aug 2021]
https://dukeghic.org/
Our Blog
No new digest content identified.
EDCTP [to 14 Aug 2021]
http://www.edctp.org/
The European & Developing Countries Clinical Trials Partnership (EDCTP) aims to accelerate the development of new or improved drugs, vaccines, microbicides and diagnostics against HIV/AIDS, tuberculosis and malaria as well as other poverty-related and neglected infectious diseases in sub-Saharan Africa, with a focus on phase II and III clinical trials
12 August 2021
Recommendations from the EDCTP/ECRIN session on clinical trial infrastructure and capacity building in Africa
Emory Vaccine Center [to 14 Aug 2021]
http://www.vaccines.emory.edu/
Vaccine Center News
No new digest content identified.
European Vaccine Initiative [to 14 Aug 2021]
http://www.euvaccine.eu/
Latest News
No new digest content identified.
FDA [to 14 Aug 2021]
https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/default.htm
Press Announcements
August 13, 2021 – Coronavirus (COVID-19) Update: August 13, 2021
:: On Thursday, FDA amended the emergency use authorizations (EUAs) for both the Pfizer-BioNTech COVID-19 Vaccine and the Moderna COVID-19 Vaccine to allow for the use of an additional dose in certain immunocompromised individuals, specifically, solid organ transplant recipients or those who are diagnosed with conditions that are considered to have an equivalent level of immunocompromise. This action does not apply to people who are not immunocompromised.
August 12, 2021 – Coronavirus (COVID-19) Update: FDA Authorizes Additional Vaccine Dose for Certain Immunocompromised Individuals
Fondation Merieux [to 14 Aug 2021]
http://www.fondation-merieux.org/
News, Events
No new digest content identified.
Gavi [to 14 Aug 2021]
https://www.gavi.org/
News Releases
13 August 2021
UK-donated COVID-19 vaccine doses reach African countries
119,200 doses of the AstraZeneca COVID-19 vaccine shared by the United Kingdom are landing in Zambia and 51,840 in the Democratic Republic of the Congo (DRC) on 13 August. 119,040 doses are due to arrive in Malawi on 14 August, 140,160 doses in Senegal on 15 August, 299,680 doses in Egypt on 16 August, and 299,520 doses are scheduled to touch down in Uganda on 18 August. The UK has pledged to share 80 million doses of COVID-19 vaccines with COVAX, as part of a broader pledge to share 100 million doses with the rest of the world. The UK Government has already committed £548 million in funding to the Gavi COVAX Advance Market Commitment.
GHIT Fund [to 14 Aug 2021]
https://www.ghitfund.org/newsroom/press
GHIT was set up in 212 with the aim of developing new tools to tackle infectious diseases that
No new digest content identified.
Global Fund [to 14 Aug 2021]
https://www.theglobalfund.org/en/news/
News & Stories
News
Global Fund grants US$37 million to FIND to advance tuberculosis prevention and control in India
13 August 2021
FIND, the global alliance for diagnostics, and the Global Fund to Fight AIDS, Tuberculosis and Malaria announced that FIND has been awarded over US$37 million to ensure the inception and continuation of three projects dedicated to advancing tuberculosis prevention and control in India: project SHAQTI; project JEET; and Unite to ACT.
Global Research Collaboration for Infectious Disease Preparedness [GloPID-R] [to 14 Aug 2021]
https://www.glopid-r.org/news/
News
No new digest content identified.
Hilleman Laboratories [to 14 Aug 2021]
http://www.hillemanlabs.org/
Website reports “under maintenance” at inquiry
Human Vaccines Project [to 14 Aug 2021]
http://www.humanvaccinesproject.org/
News
News webpage not responding at inquiry
IAVI [to 14 Aug 2021]
https://www.iavi.org/newsroom
Latest News
FEATURES
IAVI launches Leadership Development Program to strengthen scientific research in Africa and India
August 9, 2021
New leadership program positions homegrown scientists to drive research into HIV and other infectious
With support from the U.S. Agency for International Development (USAID), IAVI has commenced a Leadership Development Program (LDP) that will position African and Indian scientists to take the lead in fashioning, driving, and supporting the scientific research enterprise. The new initiative will offer opportunities to early- and mid-career scientists to develop their skills to become the next generation of science leadership in their respective fields of expertise.
LDP, in its first cohort, will support 30 participants from African and Indian Clinical Research Centers (CRCs), who range from laboratory scientists to clinical trials specialists. This first cohort includes scientifically experienced participants who have run independent science projects, as well as those who have managed only parts of much larger research enterprises. The cohort also includes recently appointed postdoctoral fellows in HIV prevention research at the respective partner CRCs…
International Coalition of Medicines Regulatory Authorities [ICMRA]
http://www.icmra.info/drupal/en/news
Selected Statements, Press Releases, Research
No new digest content identified.
ICRC [to 14 Aug 2021]
https://www.icrc.org/en/whats-new
Selected News Releases, Statements, Reports
COVID-19 vaccination: ICRC’s work in armed conflicts and hard-to-reach places
The ICRC is working with Red Cross and Red Crescent partners around the world to support COVID-19 vaccination in armed conflicts as well as hard-to-reach and volatile areas.
11-08-2021 | Article
Interactive map of our operational support for COVID-19 vaccination plan (527 KB)
map_icrc_covid19.pdf
IFFIm
http://www.iffim.org/
Press Releases/Announcements
No new digest content identified.
IFRC [to 14 Aug 2021]
http://media.ifrc.org/ifrc/news/press-releases/
Selected Press Releases, Announcements
Algeria, Middle East and North Africa, Tunisia
Combination of wildfires and COVID-19 threaten tens of thousands of lives in Algeria and Tunisia
Algeria/Tunisia/Beirut, 13 August 2021 – The wildfires currently spreading in Algeria and Tunisia threaten the lives of tens of thousands, while also damaging local ecosystems, infrastructures and livelihoods. Both countries face a multi-hazard situat …
13 August 2021
Fiji
Pacific: Young people encouraged to join the fight against the pandemic
Suva, 12 August 2021 – As global preparations take place this week to mark the celebration of International Youth Day, young people in the Pacific are urged to follow the example of Red Cross volunteers and join the battle against COVID-19, as the glob …
12 August 2021
Bangladesh
Mass COVID-19 vaccinations kick off in Bangladesh camps
Cox’s Bazar, Bangladesh, 10 August 2021: Vaccinations have begun for people in the camps for displaced people, amid a record COVID-19 surge in Bangladesh and a widening global vaccine divide. The vaccination campaign begins on 10 August with priority …
10 August 2021
Institut Pasteur [to 14 Aug 2021]
https://www.pasteur.fr/en/press-area
Press Documents
No new digest content identified.
IOM / International Organization for Migration [to 14 Aug 2021]
http://www.iom.int/press-room/press-releases
News
IOM Director General’s Statement on the Situation in Afghanistan
2021-08-10 19:32
ISC / International Science Council [to 14 Aug 2021]
ISC is a non-governmental organization with a unique global membership that brings together 40 international scientific Unions and Associations and over 140 national and regional scientific organizations including Academies and Research Councils.
https://council.science/current/
News
A new social contract must include genuine participation and partnership of Indigenous peoples in decision-making about research
09.08.2021
Daya Reddy, ISC President and Chair of the Committee for Freedom and Responsibility in Science (CFRS) has added his support to the United Nations’ call for a new social contract based on genuine participation and partnership that respects the rights, dignity and freedoms of all.
IVAC [to 14 Aug 2021]
https://www.jhsph.edu/research/centers-and-institutes/ivac/index.html
Updates; Events
No new digest content identified.
IVI [to 14 Aug 2021]
http://www.ivi.int/
Selected IVI News, Announcements, Events
IVI partners with SK bioscience to conduct late-stage global clinical trials of SK bioscience’s COVID-19 vaccine
:: IVI to conduct Phase 3 clinical trials of SK bioscience’s recombinant protein vaccine candidate (GBP510) in Europe and Southeast Asia
:: Additionally, IVI forms partnership with SK and KNIH to jointly conduct antibody testing for the global trials to accelerate development of “Wave 2” vaccines
August 10, 2021
Johns Hopkins Center for Health Security [to 14 Aug 2021]
https://www.centerforhealthsecurity.org/news/center-news/
Center News
Johns Hopkins Center for Health Security Commends U.S. Senators Baldwin, Casey, King, and Smith for Introducing the Disease X Act to Respond to Future Viral Outbreaks
August 9, 2021
MSF/Médecins Sans Frontières [to 14 Aug 2021]
http://www.msf.org/
Latest [Selected Announcements]
Coronavirus COVID-19 pandemic
Responding to COVID-19: Global Accountability Report 4 – January to April 2021
Report 12 Aug 2021
Yemen
Treating COVID-19 in Yemen amongst fear, stigma and misinformation
Project Update 11 Aug 2021
Afghanistan
As violence soars across Afghanistan access to healthcare is dangerously limited
Project Update 10 Aug 2021
National Academy of Medicine – USA [to 14 Aug 2021]
https://nam.edu/programs/
Selected News/Programs
No new digest content identified.
National Academy of Sciences – USA [to 14 Aug 2021]
http://www.nasonline.org/news-and-multimedia/
News
No new digest content identified.
National Vaccine Program Office – U.S. HHS [to 14 Aug 2021]
https://www.hhs.gov/vaccines/about/index.html
Upcoming Meetings/Latest Updates
No new digest content identified.
NIH [to 14 Aug 2021]
http://www.nih.gov/news-events/news-releases
News Releases
Monoclonal antibody prevents malaria in small NIH trial
August 11, 2021 — Additional NIH studies underway will build on encouraging early results.
NIH launches study of third COVID-19 vaccine dose in kidney transplant recipients
August 10, 2021 — Trial will assess antibody response in people who did not respond to two-dose regimen.
PATH [to 14 Aug 2021]
https://www.path.org/media-center/
Press Releases
No new digest content identified.
Sabin Vaccine Institute [to 14 Aug 2021]
http://www.sabin.org/updates/pressreleases
Statements and Press Releases
No new digest content identified.
UNAIDS [to 14 Aug 2021]
http://www.unaids.org/en
Selected Press Releases/Reports/Statements
12 August 2021
UNAIDS strongly supports calls for the rejection of draft law targeting LGBTI people in Ghana
10 August 2021
Defending rights and overcoming fear in Kyrgyzstan
UNESCO [to 14 Aug 2021]
http://en.unesco.org/news
Selected Latest News
Covid-19 and vaccination in Latin America and the Caribbean: challenges, needs and opportunities
04/08/2021 88 pages
UNESCO publishes a document that generates more evidence on the production, access and distribution of vaccines in the region, with the aim of improving existing vaccination policies, applying a human rights, open science and ethical standards approach
The report (available in Spanish) studies the vaccination plans of all Latin American and Caribbean countries and analyzes the region’s capacity to produce vaccines against Covid-19.
UNESCO has called for considering the vaccine as a universal public good and ensuring its equitable, affordable and timely access. In addition, they have insisted on redoubling scientific and technological cooperation to accelerate vaccine production and thus guarantee access to as many people as possible in the shortest time possible.
PDF: https://unesdoc.unesco.org/ark:/48223/pf0000378377
Ethical principles to guide COVID-19 vaccination campaign in Africa
03/08/2021 Fact Sheet :: 2 pages
PDF: https://en.unesco.org/sites/default/files/factsheet.pdf
Key Ethical Considerations
The Ubuntu principle – sense of the Community – is of vital importance – it is vital landmark to guide actions.
Promotion of Universal Access to a Public Good. Avoid Vaccine Nationalism and Predatory Rush.
Protection from vulnerability must be applied to observe fairness within and between countries.
Public-Private Partnerships are required, to observe Equality, Justice, and Solidarity. The development of vaccines by the pharmaceutical industry was also supported by public funds, often in collaboration with public academic institutions.
Public Trust and people-centred approach are key in reaching herd immunity. Trust can only grow from a respectful dialogue, Public Transparency, Critical and Open Deliberations on the scientific practices
behind vaccines, and as well as on public policies on vaccination.
UNHCR Office of the United Nations High Commissioner for Refugees [to 14 Aug 2021]
http://www.unhcr.org/en-us/media-centre.htmlS
Selected News Releases, Announcements
UNHCR concerned over U.S. expulsion flights under COVID-19 asylum restrictions
11 Aug 2021
UNICEF [to 14 Aug 2021]
https://www.unicef.org/media/press-releases
Press Releases, News Notes, Statements [Selected]
Press release
08/14/2021
UNICEF set to reach children and families in Haiti with humanitarian assistance in the aftermath of powerful earthquake
Unitaid [to 14 Aug 2021]
https://unitaid.org/
Featured News
Digital technologies support patients with treatment from a distance, allowing more flexibility in care
Geneva, 11 August 2021 – As the Unitaid-funded ASCENT project launches the main research phase, investigating the effectiveness of digital adherence technologies and data-driven support interventions on TB treatment completion and success rates, we asked the researchers leading the study to tell us more about their work and its potential impact.
Three digital adherence technologies – smart pillboxes, video supported treatment and medication labels or sleeves – connect patients remotely to health centres, allowing patients flexibility in their treatment while ensuring clinics can track patients’ adherence and provide tailored care where needed…
Vaccine Equity Initiative [to 14 Aug 2021]
https://vaccineequitycooperative.org/news/
News
No new digest content identified.
Vaccination Acceptance & Demand Initiative [Sabin) [to 14 Aug 2021]
https://www.vaccineacceptance.org/
Announcements
No new digest content identified.
Vaccine Confidence Project [to 14 Aug 2021]
http://www.vaccineconfidence.org/
News, Research and Reports
Coronavirus global impact
Launched April 2, 2020 and recurring every 3 days, Premise Data is utilizing its global network of Contributors to assess economic, social, and health sentiment surrounding the coronavirus (COVID-19).
MacArthur Grant
Our work on the crucial role confidence and cooperation plays in recovery from the COVID-19 pandemic in 2021 caught the attention of the MacArthur Foundation. The foundation has given The Confidence Project a major grant so it can begin this work in a focused and specific way…
Vaccine Education Center – Children’s Hospital of Philadelphia [to 14 Aug 2021]
http://www.chop.edu/centers-programs/vaccine-education-center
News
No new digest content identified.
Wellcome Trust [to 14 Aug 2021]
https://wellcome.ac.uk/news
News and reports
Explainer
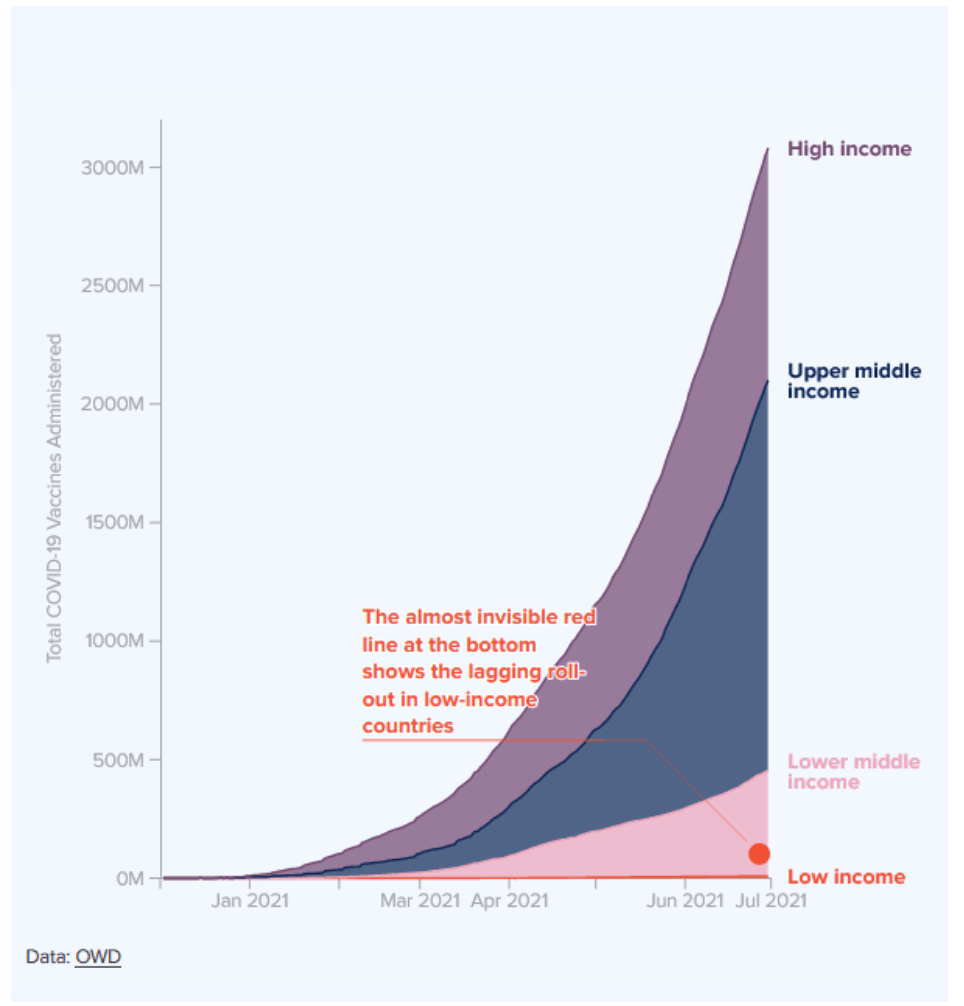
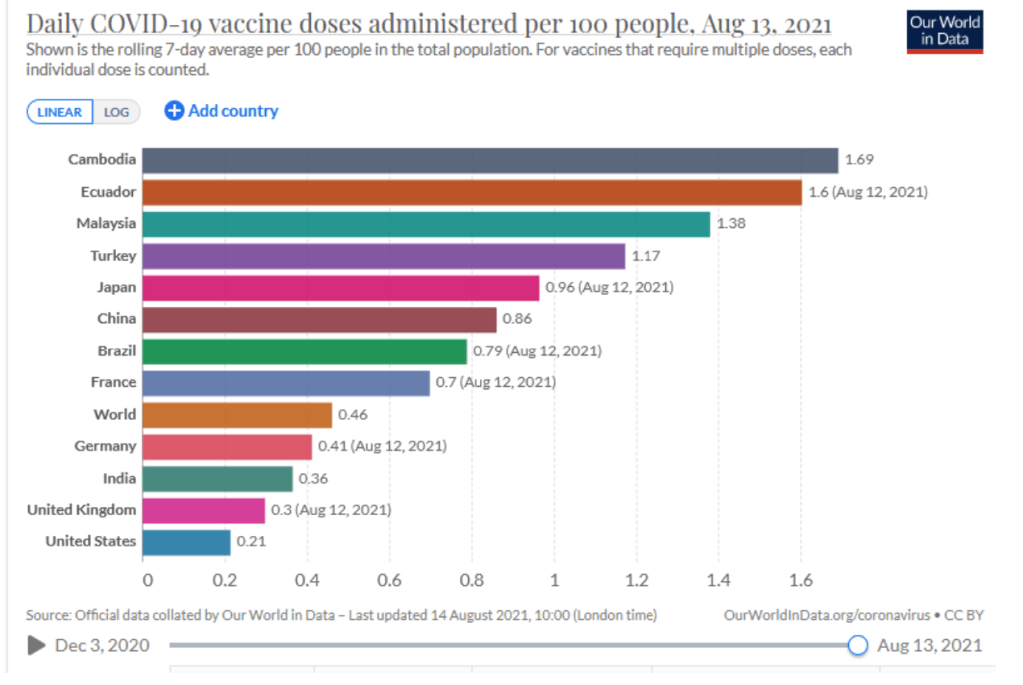
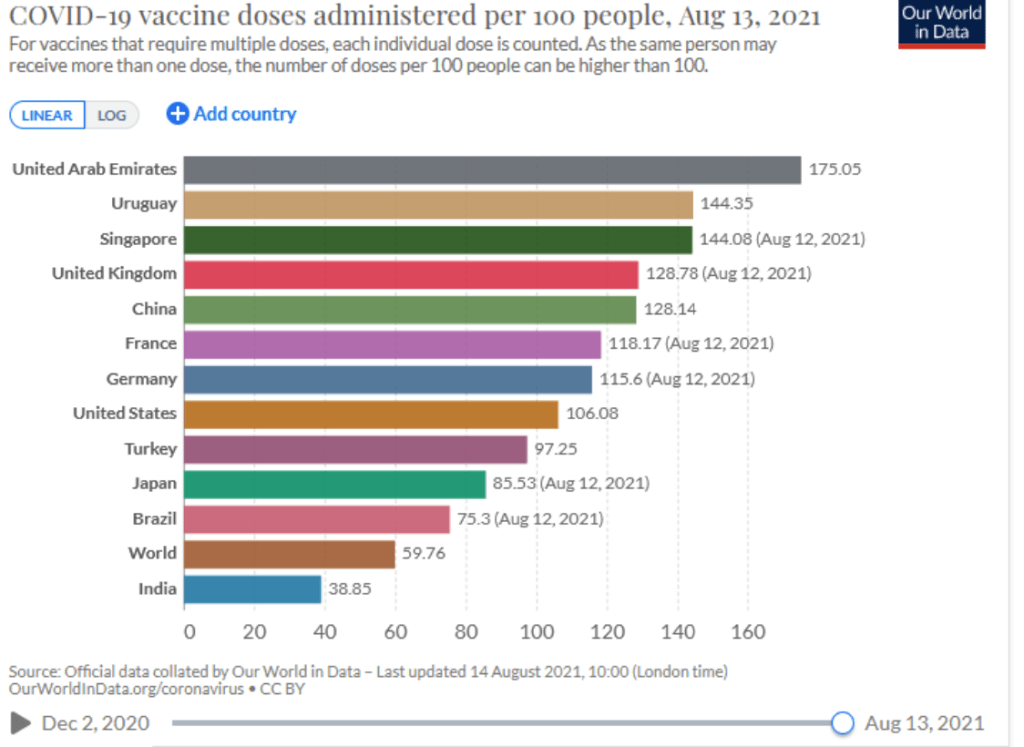
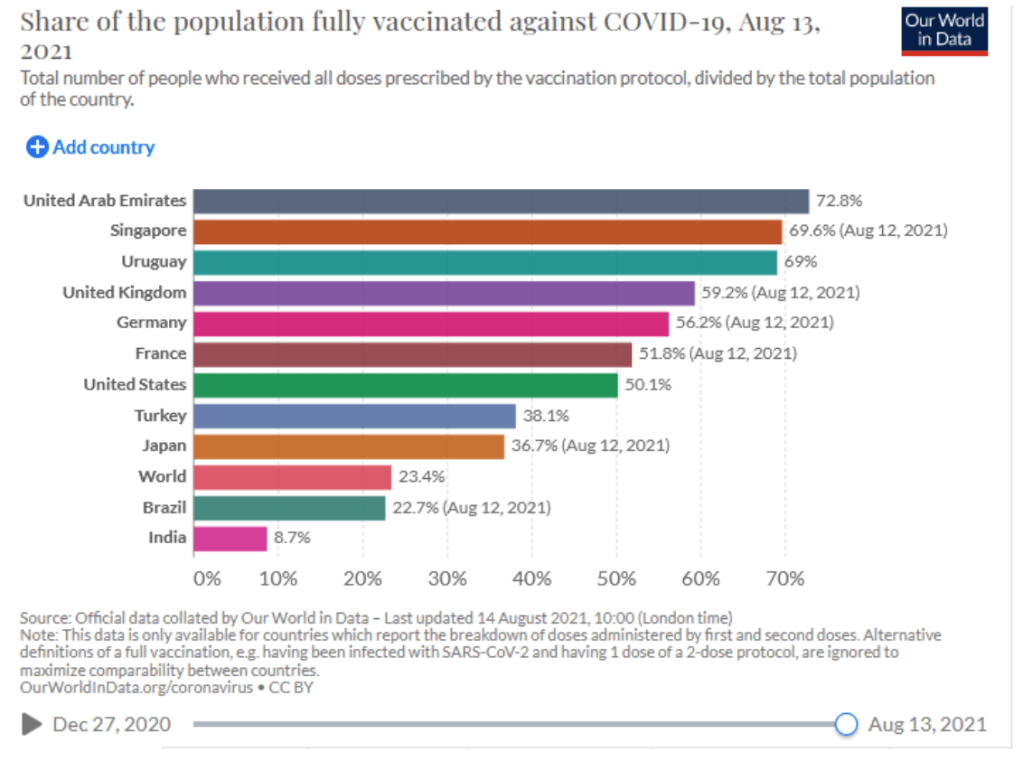
When will the world be vaccinated against Covid-19?
10 August 2021
How should the global community prioritise the distribution of Covid-19 vaccines to give us the best chance of ending the pandemic and saving lives? Could booster vaccines impact global roll out?
The Wistar Institute [to 14 Aug 2021]
https://www.wistar.org/news/press-releases
Press Releases
No new digest content identified.
WFPHA: World Federation of Public Health Associations [to 14 Aug 2021]
https://www.wfpha.org/
Latest News
No new digest content identified.
World Bank [to 14 Aug 2021]
http://www.worldbank.org/en/news/all
Selected News, Announcements
Nepal to Get 4 Million Doses of Moderna Vaccines through COVAX
KATHMANDU, August 13, 2021 – Nepal is the second country globally to have completed agreements with GAVI to procure 4 million doses of Moderna vaccines, financed by the World Bank, through the COVAX…
Date: August 13, 2021 Type: Press Release
World Customs Organization – WCO [to 14 Aug 2021]
http://www.wcoomd.org/
Latest News – Selected Items
11 August 2021
WCO Secretary General addresses UPU Ministerial Conference
World Organisation for Animal Health (OIE) [to 14 Aug 2021]
https://www.oie.int/en/for-the-media/press-releases/2021/
Press Releases, Statements
No new digest content identified.
WTO – World Trade Organisation [to 14 Aug 2021]
http://www.wto.org/english/news_e/news_e.htm
WTO News and Events
DDG González underscores importance of women’s economic empowerment in trade
9 August 2021
In an opening address to a joint webinar marking the launch of a research and outreach project on “Gender and Trade in the Americas” on 3 August, Deputy Director-General Anabel González emphasized the central role of women in economic and social lives and the WTO´s work in supporting gender responsive trade policies. The webinars were organized by member institutions of the WTO Chairs Programme in Barbados, Chile, and Mexico with project funding provided by the Netherlands.
::::::
ARM [Alliance for Regenerative Medicine] [to 14 Aug 2021]
Press Releases – Alliance for Regenerative Medicine (alliancerm.org)
Selected Press Releases
No new digest content identified.
BIO [to 14 Aug 2021]
https://www.bio.org/press-releases
Press Releases, Letters, Testimony, Comments [Selected]
Biden Drug Plan Adds Barriers to Seniors Accessing Lifesaving Medicines
August 12, 2021
President Joe Biden today announced a slew of drug pricing reforms, including changes that would radically alter the widely successful Medicare program for seniors and people with disabilities. Rich Masters, BIO’s Chief Public Affairs and Advocacy…
DCVMN – Developing Country Vaccine Manufacturers Network [to 14 Aug 2021]
http://www.dcvmn.org/
News; Upcoming events
No new digest content identified.
ICBA – International Council of Biotechnology Associations [to 14 Aug 2021]
https://internationalbiotech.org/news/
News
No new digest content identified.
IFPMA [to 14 Aug 2021]
http://www.ifpma.org/resources/news-releases/
Selected Press Releases, Statements, Publications
No new digest content identified.
International Generic and Biosimilar Medicines Association [IGBA]
https://www.igbamedicines.org/
News
No new digest content identified.
International Alliance of Patients’ Organizations – IAPO [to 14 Aug 2021]
https://www.iapo.org.uk/news/topic/6
Press and media [Selected]
No new digest content identified.
PhRMA [to 14 Aug 2021]
http://www.phrma.org/
Latest News [Selected]
PhRMA Statement on President Biden’s Remarks to Lower Prescription Drug Prices
August 12, 2021
Pharmaceutical Research and Manufacturers of America president and CEO Stephen J. Ubl issued the following statement today after President Biden’s call on Congress to lower prescription drug prices.
“We stand ready to work with lawmakers and do our part so that patients can see lower costs at the pharmacy and continued access to the cures and treatments they need. Unfortunately, the policies the president outlined today would undermine access to life-saving medicines and fail to address an insurance system that shifts the cost of treatments onto vulnerable patients…