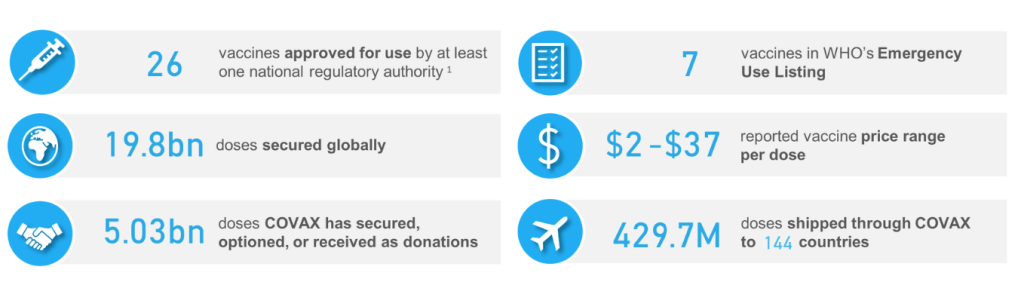
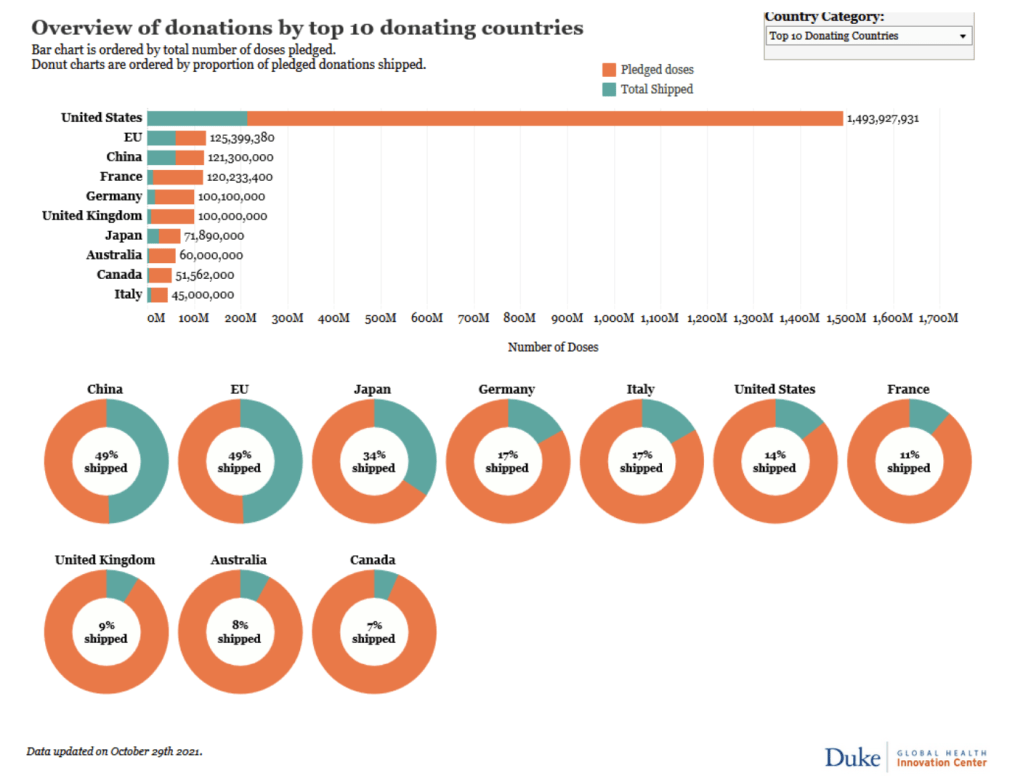
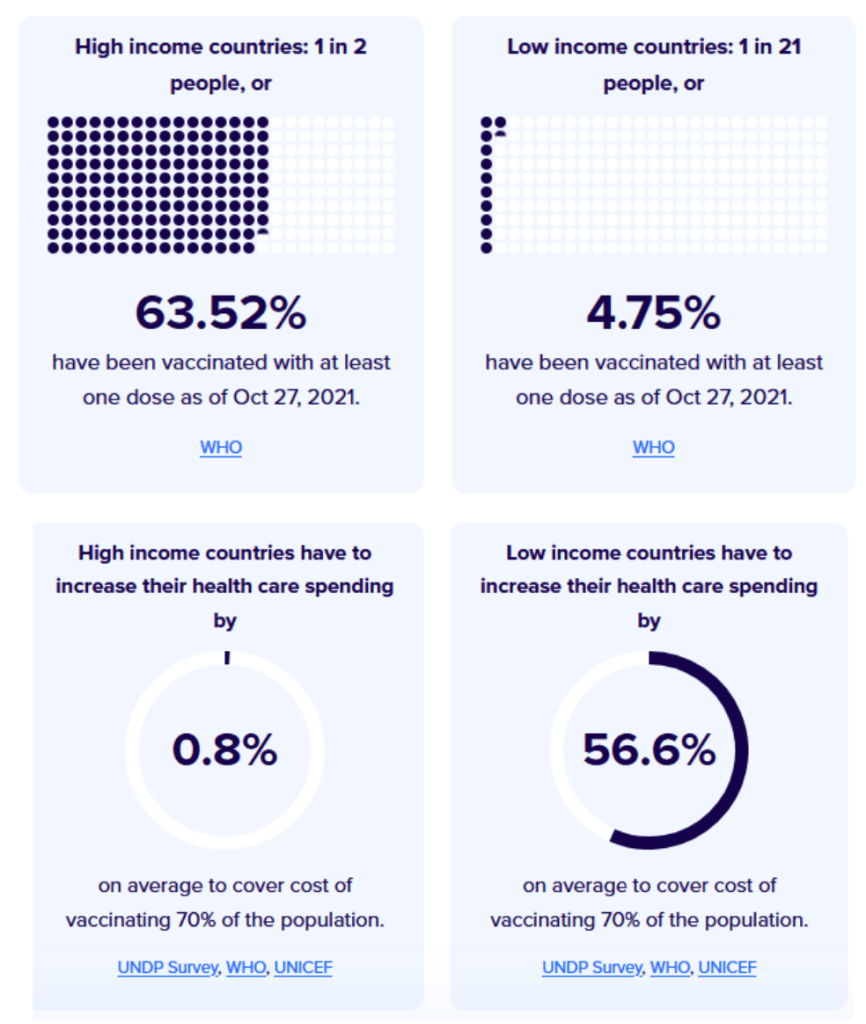
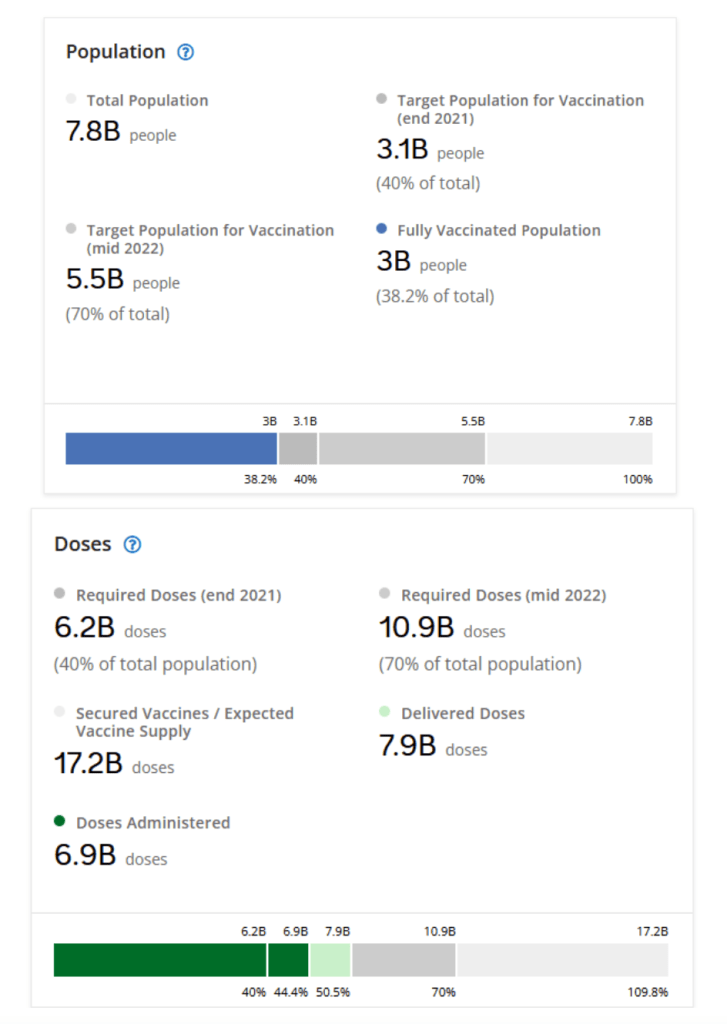
::::::
Organization Announcements
Editor’s Note:
Careful readers will note that the number and range of organizations now monitored in our Announcements section below has grown as the impacts of the pandemic have spread across global economies, supply chains and programmatic activity of multilateral agencies and INGOs.
Paul G. Allen Frontiers Group [to 30 Oct 2021]
https://alleninstitute.org/news-press/
News
No new digest content identified.
BARDA – U.S. Department of HHS [to 30 Oct 2021]
https://www.phe.gov/about/barda/Pages/default.aspx
News
No new digest content identified.
BMGF – Gates Foundation [to 30 Oct 2021]
https://www.gatesfoundation.org/ideas/media-center
Press Releases and Statements
Bill & Melinda Gates Medical Research Institute [to 30 Oct 2021]
https://www.gatesmri.org/
The Bill & Melinda Gates Medical Research Institute is a non-profit biotech organization. Our mission is to develop products to fight malaria, tuberculosis, and diarrheal diseases—three major causes of mortality, poverty, and inequality in developing countries. The world has unprecedented scientific tools at its disposal; now is the time to use them to save the lives of the world’s poorest people
No new digest content identified.
CARB-X [to 30 Oct 2021]
https://carb-x.org/
News
No new digest content identified.
Center for Vaccine Ethics and Policy – GE2P2 Global Foundation [to 30 Oct 2021]
https://centerforvaccineethicsandpolicy.net/
News/Analysis/Statements
:: Past weekly editions and posting of all segments of Vaccines and Global Health: The Week in Review are available here.
:: [NEW] Informed Consent: A Monthly Review – October 2021 is now posted here
CEPI – Coalition for Epidemic Preparedness Innovations [to 30 Oct 2021]
http://cepi.net/
Latest News
Statement from Dr Richard Hatchett, CEO of CEPI, following the G20 Health & Finance Ministers meeting
The G20 must redouble their efforts to end the COVID-19 pandemic and commit funding and leadership to prepare the world for future pandemic threats.
End Pandemics
29 Oct 2021
Unleashing the power of science to work for everyone everywhere
With the right investments, leaders of the G20 nations can help CEPI and the world prepare for almost any pathogen – old or new, known or unknown – that we might encounter.
COVID-19
28 Oct 2021
Vaccine production efforts across key regions mapped in first-of-its-kind study to prepare for future pandemics
CEPI’s landscaping exercise revealed vaccine manufacturing capacity needs to be expanded and diversified in Africa, Latin America-Caribbean, the Middle East, as well as further improvements in South East Asia-Western Pacific.
Blog
27 Oct 2021
DARPA – Defense Advanced Research Projects Agency [to 30 Oct 2021
https://www.darpa.mil/news
News
No new digest content identified.
Duke Global Health Innovation Center [to 30 Oct 2021]
https://dukeghic.org/
Our Blog
No new digest content identified.
EDCTP [to 30 Oct 2021]
http://www.edctp.org/
The European & Developing Countries Clinical Trials Partnership (EDCTP) aims to accelerate the development of new or improved drugs, vaccines, microbicides and diagnostics against HIV/AIDS, tuberculosis and malaria as well as other poverty-related and neglected infectious diseases in sub-Saharan Africa, with a focus on phase II and III clinical trials
News
No new digest content identified.
Emory Vaccine Center [to 30 Oct 2021]
http://www.vaccines.emory.edu/
Vaccine Center News
No new digest content identified.
European Vaccine Initiative [to 30 Oct 2021]
http://www.euvaccine.eu/
Latest News
No new digest content identified.
Fondation Merieux [to 30 Oct 2021]
http://www.fondation-merieux.org/
News, Events
No new digest content identified.
Gavi [to 30 Oct 2021]
https://www.gavi.org/
News releases
28 October 2021
Combining vaccines with nutrition: a game-changer against COVID-19 and future pandemics say Gavi and SUN
Malnutrition and infectious diseases together cause millions of preventable child deaths every year and contribute to a vicious cycle of poor health, stunted growth, poverty and exclusion
Rolling out immunisation and nutrition programmes together significantly increases the number of people reached and reduces delivery costs
Gavi, the Vaccine Alliance, and the Scaling Up Nutrition (SUN) Movement have now partnered to launch this innovative two-pronged healthcare approach
This approach is a game-changer in supporting communities to be resilient to future pandemics, especially for vulnerable people including women and children
GHIT Fund [to 30 Oct 2021]
https://www.ghitfund.org/newsroom/press
GHIT was set up in 2012 with the aim of developing new tools to tackle infectious diseases that
No new digest content identified.
Global Fund [to 30 Oct 2021]
https://www.theglobalfund.org/en/news/
News & Stories
No new digest content identified.
Global Research Collaboration for Infectious Disease Preparedness [GloPID-R] [to 30 Oct 2021]
https://www.glopid-r.org/news/
News
No new digest content identified.
Hilleman Laboratories [to 30 Oct 2021]
http://www.hillemanlabs.org/
Website reports “under maintenance” at inquiry
Human Vaccines Project [to 30 Oct 2021]
http://www.humanvaccinesproject.org/
News
HVP COVID Report
Oct 28, 2021
Allison Greaney: Vaccines Are Holding up Against Delta, but What Will Come Next?
IAVI [to 30 Oct 2021]
https://www.iavi.org/newsroom
Latest News
PRESS RELEASES
IAVI and the Biomedical Advanced Research and Development Authority partner to advance filovirus vaccine candidates
NEW YORK – OCTOBER 27, 2021 – IAVI announced today the award of up to US$126 million from the Biomedical Advanced Research and Development Authority (BARDA) at the U.S. Department of Health and Human Services to develop two recombinant vesicular stomatitis virus (rVSV)-vectored filovirus vaccine candidates. This award supports preclinical activities and includes options for clinical development up to and inclusive of a Phase II clinical trial of IAVI’s rVSV Sudan ebolavirus vaccine candidate (rVSVΔG-SUDV-GP). Optional work that would continue the development of IAVI’s Marburg virus vaccine candidate (rVSVΔG-MARV-GP) that is currently supported by the Defense Threat Reduction Agency of the U.S. Department of Defense could be funded at a later date.
No licensed vaccines against Sudan ebolavirus (SUDV) or Marburg virus (MARV) are currently available…
International Coalition of Medicines Regulatory Authorities [ICMRA]
http://www.icmra.info/drupal/en/news
Selected Statements, Press Releases, Research
No new digest content identified.
ICRC [to 30 Oct 2021]
https://www.icrc.org/en/whats-new
Selected News Releases, Statements, Reports
Actions must speak louder than words: 5 asks to achieve equity in vaccine delivery
A people’s vaccine should protect the affluent and the poor, the elderly as well as the young, forcibly displaced persons, migrants regardless of their immigration status, and other often neglected populations, both in urban areas and in rural commun
28-10-2021 | Statement
[See COVID above for detail]
When conflict and climate change collide – ICRC warning ahead of COP26
Bamako/Geneva (ICRC) – Climate change is everywhere, but in Mali, a country battered by nearly a decade of conflict, it is pushing families to the brink.Hawa Dicko told me in a dusty, sprawling displacement camp how she lost her home twice – first be
28-10-2021 | News release
ICRC President Peter Maurer: Education for front-line communities must be a priority
On the occasion of the 4th International Conference on the Safe Schools Declaration, which took place from 25 to 27 October 2021 in Abuja, Nigeria, ICRC President Peter Maurer delivered the following pre-recorded message in the closing segment.
27-10-2021 | Statement
IFFIm
http://www.iffim.org/
Press Releases/Announcements
No new digest content identified.
IFRC [to 30 Oct 2021]
http://media.ifrc.org/ifrc/news/press-releases/
Selected Press Releases, Announcements
28/10/2021
Red Cross Red Crescent report reveals extent of the impact on people forced to flee their homes by floods, fires and drought around the world
25/10/2021
Urgent action needed as COVID-19 overwhelms PNG h\health system
Institut Pasteur [to 30 Oct 2021]
https://www.pasteur.fr/en/press-area
Press Documents
No new digest content identified.
IOM / International Organization for Migration [to 30 Oct 2021]
http://www.iom.int/press-room/press-releases
News – Selected
News 29 Oct 2021
Niger’s Leadership on Migration Acknowledged During High-Level IOM Visit
News 29 Oct 2021
IOM Launches Institutional Strategy on Migration, Environment and Climate Change for Next Decade
News 29 Oct 2021
Open Letter to G20 Heads of State and Government – An Appeal to Make Vaccines Accessible to People on the Move
[See COVID above for detail]
News 29 Oct 2021
IOM Marks 60 Years of International Migration Journal
ISC / International Science Council [to 30 Oct 2021]
https://council.science/current/
ISC is a non-governmental organization with a unique global membership that brings together 40 international scientific Unions and Associations and over 140 national and regional scientific organizations including Academies and Research Councils.
Blog
International scientific advisors call for science and innovation to be at the centre of action on climate change
29.10.2021
In a statement released by the UK Government Office for Science, senior scientific advisors from around the world, including members of the ISC’s Governing Board, and network of affliated bodies and committees, as well as representatives of the ISC Membership, call for ambitious, science-based strategies to ‘keep the 1.5°C temperature goal alive’.
News
Members of International Science Council commit to work for change in scientific publishing, and endorse eight principles for reform
25.10.2021
As the 2021 Open Access week begins, the scientific community as represented by the International Science Council’s members has approved a resolution committing to work to reform scholarly publishing, and to endorse eight fundamental principles for scientific publishing that contributes to the advancement of science as a global public good.
IVAC [to 30 Oct 2021]
https://www.jhsph.edu/research/centers-and-institutes/ivac/index.html
Updates; Events
No new digest content identified.
IVI [to 30 Oct 2021]
http://www.ivi.int/
IVI News & Announcements
IVI and JEDI partner for innovations in health and science
October 25, 2021 – SEOUL, Republic of Korea, PARIS, France, BRUSSELS, Belgium, BERLIN, Germany – The International Vaccine Institute (IVI) and the Joint European Disruptive Initiative (JEDI) signed a Memorandum of Understanding to establish a collaborative relationship. Both organizations are dedicated to advancing innovations in health and science. IVI and JEDI will explore many cooperation routes, in particular around innovative approaches to zoonoses, infectious diseases and in addressing the global threat of antimicrobial resistance (AMR)…
Johns Hopkins Center for Health Security [to 30 Oct 2021]
https://www.centerforhealthsecurity.org/news/center-news/
Center News
New Report: Navigating the World that COVID-19 Made: A Strategy for Revamping the Pandemic Research and Development Preparedness and Response Ecosystem
October 29, 2021
[See COVID above for detail]
MSF/Médecins Sans Frontières [to 30 Oct 2021]
http://www.msf.org/
Latest [Selected Announcements]
Haiti
Haiti fuel crisis severely limits access to vital medical care
Press Release 27 Oct 2021
Mediterranean migration
Nearly 400 people rescued from the Mediterranean need a place of safety
Project Update 26 Oct 2021
National Academy of Medicine – USA [to 30 Oct 2021]
https://nam.edu/programs/
Selected News/Programs/Events
No new digest content identified.
National Academy of Sciences – USA [to 30 Oct 2021]
http://www.nasonline.org/news-and-multimedia/
News
No new digest content identified.
National Vaccine Program Office – U.S. HHS [to 30 Oct 2021]
https://www.hhs.gov/vaccines/about/index.html
Upcoming Meetings/Latest Updates
No new digest content identified.
NIH [to 30 Oct 2021]
http://www.nih.gov/news-events/news-releases
News Releases
NIH researchers identify how two people controlled HIV after stopping treatment
October 29, 2021 — Different mechanisms suppressed the virus in each person.
NIH, FDA and 15 private organizations join forces to increase effective gene therapies for rare diseases
October 27, 2021 — While there are approximately 7,000 rare diseases, only two heritable diseases currently have FDA-approved gene therapies.
The National Institutes of Health, U.S. Food and Drug Administration, 10 pharmaceutical companies and five non-profit organizations have partnered to accelerate development of gene therapies for the 30 million Americans who suffer from a rare disease. While there are approximately 7,000 rare diseases, only two heritable diseases currently have FDA-approved gene therapies. The newly launched Bespoke Gene Therapy Consortium (BGTC), part of the NIH Accelerating Medicines Partnership (AMP) program and project-managed by the Foundation for the National Institutes of Health (FNIH), aims to optimize and streamline the gene therapy development process to help fill the unmet medical needs of people with rare diseases.
“Most rare diseases are caused by a defect in a single gene that could potentially be targeted with a customized or ‘bespoke’ therapy that corrects or replaces the defective gene,” said NIH Director Francis S. Collins, M.D., Ph.D. “There are now significant opportunities to improve the complex development process for gene therapies that would accelerate scientific progress and, most importantly, provide benefit to patients by increasing the number of effective gene therapies.”…
NIH awards nearly $75M to catalyze data science research in Africa
October 26, 2021 — New program will establish data science research and training network across the continent.
The National Institutes of Health is investing about $74.5 million over five years to advance data science, catalyze innovation and spur health discoveries across Africa. Under its new Harnessing Data Science for Health Discovery and Innovation in Africa (DS-I Africa) program, the NIH is issuing 19 awards to support research and training activities. DS-I Africa is an NIH Common Fund program that is supported by the Office of the Director and 11 NIH Institutes, Centers and Offices.
Awards will establish a consortium consisting of a data science platform and coordinating center, seven research hubs, seven data science research training programs and four projects focused on studying the ethical, legal and social implications of data science research. Awardees have a robust network of partnerships across the African continent and in the United States, including numerous national health ministries, nongovernmental organizations, corporations, and other academic institutions…
PATH [to 30 Oct 2021]
https://www.path.org/media-center/
Press Releases
No new digest content identified.
Sabin Vaccine Institute [to 30 Oct 2021]
http://www.sabin.org/updates/pressreleases
Statements and Press Releases
No new digest content identified.
UNAIDS [to 30 Oct 2021]
http://www.unaids.org/en
Selected Press Releases/Reports/Statements
31 October 2021
Western and central Africa HIV summit
29 October 2021
Brandy Rodriguez leaves legacy of courageous advocacy and community support
29 October 2021
Zero Discrimination Platform relaunched in Central African Republic
29 October 2021
Getting unconditional cash to marginalized households during COVID-19
28 October 2021
Bangkok Metropolitan Administration receives award for innovations on PrEP and key population-led services
26 October 2021
ASEAN cities protecting the gains of the HIV response during the COVID-19 pandemic
25 October 2021
Financial shortfalls hold back the HIV response in western and central Africa
UNHCR Office of the United Nations High Commissioner for Refugees [to 30 Oct 2021]
http://www.unhcr.org/en-us/media-centre.htmlS
Selected News Releases, Announcements
Open letter to G20 Heads of State and Government – UNHCR, IOM & WHO
An appeal to G20 leaders to make vaccines accessible to people on the move
29 Oct 2021
[See COVID above for detail]
Actions must speak louder than words: five asks to achieve equity in vaccine delivery
Joint UN/ Red Cross/Red Crescent Statement
28 Oct 2021
[See COVID above for detail]
New data visualization on COVID-19 eviction and homelessness risks for Venezuelan refugees and migrants
25 Oct 2021
UNICEF [to 30 Oct 2021]
https://www.unicef.org/media/press-releases
Press Releases, News Notes, Statements [Selected]
Press release
10/28/2021
New ACT-Accelerator strategy calls for US$ 23.4 billion international investment to solve inequities in global access to COVID-19 vaccines, tests & treatments
Joint statement from the ACT-Accelerator partners
[See COVID above for detail]
Statement
10/27/2021
Urgent action needed now to ensure sufficient COVID vaccine syringe supply to meet 2022 vaccination targets
Increased demand, supply chain disruptions, and ‘syringe nationalism’ could lead to significant challenges in 2022 without immediate action
[See COVID above for detail]
Press release
10/27/2021
G20 members have received 15 times more COVID-19 vaccine doses per capita than sub-Saharan African countries
Ahead of the G20 Leaders’ Summit this weekend, 48 UNICEF Africa ambassadors and supporters unite, calling on countries to deliver doses by December.
[See COVID above for detail]
Remarks
10/25/2021
Opening remarks for UNICEF virtual event on the ‘Role of Women in Polio Eradication’ – 25 Oct 2021
UNICEF Executive Director Henrietta Fore
Remarks
10/25/2021
Keynote Speech to the World Health Summit 2021 – 24 October 2021
UNICEF Executive Director Henrietta Fore
Unitaid [to 30 Oct 2021]
https://unitaid.org/
Featured News
29 October 2021
Unitaid statement at the G20 Joint Finance and Health Ministers’ Meeting
27 October 2021
WHO-Unitaid statement on the MPP licensing agreement for molnupiravir
[See COVID above for detail]
Vaccine Equity Cooperative [nee Initiative] [to 30 Oct 2021]
https://vaccineequitycooperative.org/news/
News
No new digest content identified.
Vaccination Acceptance & Demand Initiative [Sabin) [to 30 Oct 2021]
https://www.vaccineacceptance.org/
Announcements
No new digest content identified.
Vaccine Confidence Project [to 30 Oct 2021]
http://www.vaccineconfidence.org/
News, Research and Reports
Coronavirus global impact
Launched April 2, 2020 and recurring every 3 days, Premise Data is utilizing its global network of Contributors to assess economic, social, and health sentiment surrounding the coronavirus (COVID-19).
Vaccine Education Center – Children’s Hospital of Philadelphia [to 30 Oct 2021]
http://www.chop.edu/centers-programs/vaccine-education-center
News
Vaccine Update Newsletter – October 2021
Vaccine Update is our monthly email newsletter that will keep you up to date on current vaccine-related issues.
Wellcome Trust [to 30 Oct 2021]
https://wellcome.ac.uk/news
News and reports
Explainer
What treatments are working for Covid-19?
28 October 2021
From existing antivirals to new antibody therapies – researchers are working tirelessly to find the best drugs to treat Covid-19.
Explainer
Four reasons why health must be at the heart of climate action
25 October 2021
Climate change is one of the most urgent health challenges of our time. Ahead of COP26, we outline some of the damaging effects of climate change on health, and why climate action cannot ignore the evidence.
The Wistar Institute [to 30 Oct 2021]
https://www.wistar.org/news/press-releases
Press Releases
No new digest content identified.
WFPHA: World Federation of Public Health Associations [to 30 Oct 2021]
https://www.wfpha.org/
Latest News
No new digest content identified.
World Bank [to 30 Oct 2021]
http://www.worldbank.org/en/news/all
Selected News, Announcements
Joint Statement of the Multilateral Leaders Taskforce on the Strategies to Accelerate the Supply and Deployment of COVID-19 Vaccines Following its Fifth Meeting
ROME, October 30, 2021 — The heads of the International Monetary Fund, World Bank Group, World Health Organization and World Trade Organization met to discuss strategies to accelerate the…
Date: October 30, 2021 Type: Statement
[See COVID above for detail]
Remarks by World Bank Group President David Malpass to the G20 Leaders’ Summit – Session I: Global Economy and Health
Thank you, Prime Minister Draghi. The developing world faces multiple severe problems. The pandemic and the scarcity of COVID-19 vaccines are the most immediate. In addition, the recovery is being undercut…
Date: October 30, 2021 Type: Speeches and Transcripts
[See COVID above for detail]
Working towards Universal Health Coverage in East Asia and the Pacific
Highlights – Countries in East Asia and the Pacific have committed to providing universal health coverage for their populations – so they can access quality health care when they need it without falling into…
Date: October 29, 2021 Type: Brief
World Bank: Pandemic Threatens to Drive Unprecedented Number of Children into Learning Poverty
WASHINGTON D.C., October 29, 2021— The COVID-19 pandemic could drive up learning poverty, the share of 10-year-olds who cannot read a basic text, to around 70 percent in low- and middle-income countries…
Date: October 29, 2021 Type: Press Release
Remarks by World Bank Group President David Malpass at the G20 Joint Finance and Health Ministerial
Rome – October 29, 2021 Thank you, Ministers Franco and Speranza. I welcome the Italian G20 presidency’s efforts to improve coordination on pandemic preparedness and response. As we enter the third year…
Date: October 29, 2021 Type: Speeches and Transcripts
World Customs Organization – WCO [to 30 Oct 2021]
http://www.wcoomd.org/
Latest News – Selected Items
29 October 2021
Release of the Harmonized System Committee’s working documents on WCO public database
29 October 2021
The annual International Customs Forum was held in Moscow
28 October 2021
Latest edition of WCO News now available
World Organisation for Animal Health (OIE) [to 30 Oct 2021]
https://www.oie.int/en/media/news/
Press Releases, Statements
Supporting investment decisions for animal health
28 October 2021
Given that we live in a world of interconnectedness, the importance of data and improving datasets is paramount to achieving evidence-based policy-making at international and national levels. The Global Burden of Animal Diseases (GBADs) programme will act as an essential piece of a bigger digital transformation at the World Organisation for Animal Health (OIE) and it will act in complementarity with other OIE datasets and workstreams such as the Training Platform , Observatory and PVS Pathway.
WTO – World Trade Organisation [to 30 Oct 2021]
http://www.wto.org/english/news_e/news_e.htm
WTO News and Events
New WTO report on G20 shows restraint in new pandemic-related trade restrictions
28 October 2021
G20 economies have continued to roll back COVID-19-related trade-restrictive measures and demonstrated restraint in the imposition of new ones, but the value of trade covered by pandemic-related restrictions still in place now exceeds that of trade-facilitating measures, according to the latest WTO Trade Monitoring Report on G20 trade measures released today (28 October). Ahead of a G20 leaders’ summit in Rome this weekend, Director-General Ngozi Okonjo-Iweala called on G20 economies to continue to unwind pandemic-related trade restrictions and to push for a strong WTO response to the pandemic at the 12th Ministerial Conference.
::::::
ARM [Alliance for Regenerative Medicine] [to 30 Oct 2021]
https://alliancerm.org/press-releases/
Selected Press Releases
The Alliance for Regenerative Medicine Announces Election of 2022 Officers, Executive Committee, and Board of Directors
Washington, DC – October 21, 2021
BIO [to 30 Oct 2021]
https://www.bio.org/press-releases
Press Releases, Letters, Testimony, Comments [Selected]
No new digest content identified.
DCVMN – Developing Country Vaccine Manufacturers Network [to 30 Oct 2021]
http://www.dcvmn.org/
News; Upcoming events
No new digest content identified.
ICBA – International Council of Biotechnology Associations [to 30 Oct 2021]
https://internationalbiotech.org/news/
News
No new digest content identified.
IFPMA [to 30 Oct 2021]
http://www.ifpma.org/resources/news-releases/
Selected Press Releases, Statements, Publications
Bolstering action against future pandemics: pharma policy perspectives on delivering medical countermeasures
25 October 2021
:: Future pandemic preparedness discussions cannot and must not overshadow the need for urgent joint action to redistribute COVID-19 vaccines through COVAX and donations.
:: Planning for better pandemic preparedness requires measures to develop effective and safe pandemic products even faster to save lives and livelihoods, which requires first pathogen sharing, second, strong incentive frameworks and third, a sustainable innovation ecosystem.
:: To ensure equitable access to those products for people worldwide requires more effective collaboration with governments, multilateral organizations, regulators, and other companies and sectors.
[See Milestones/Perspective above for detail]
International Generic and Biosimilar Medicines Association [IGBA]
https://www.igbamedicines.org/
News
No new digest content identified.
International Alliance of Patients’ Organizations – IAPO [to 30 Oct 2021]
https://www.iapo.org.uk/news/topic/6
Press and media [Selected]
No new digest content identified.
PhRMA [to 30 Oct 2021]
http://www.phrma.org/
Latest News [Selected]
No new digest content identified.