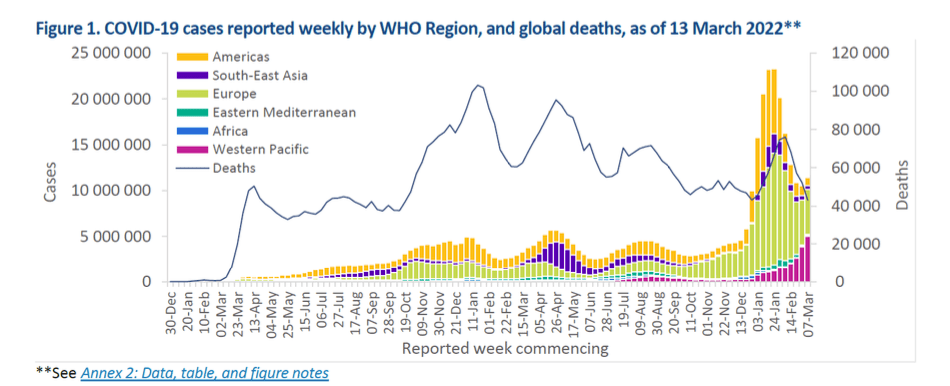
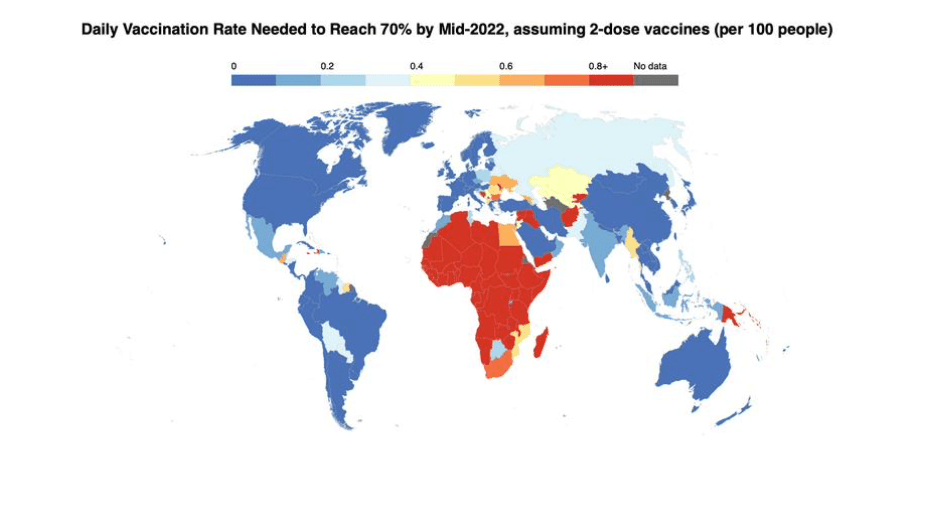
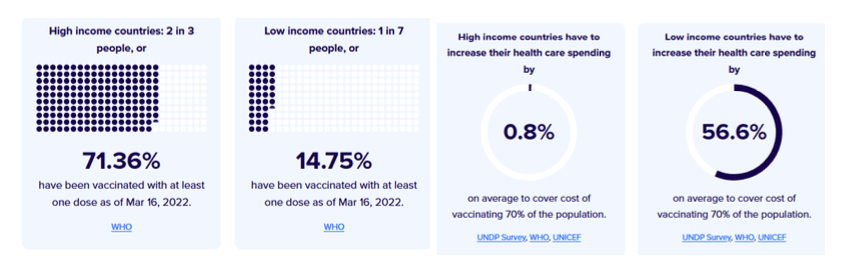
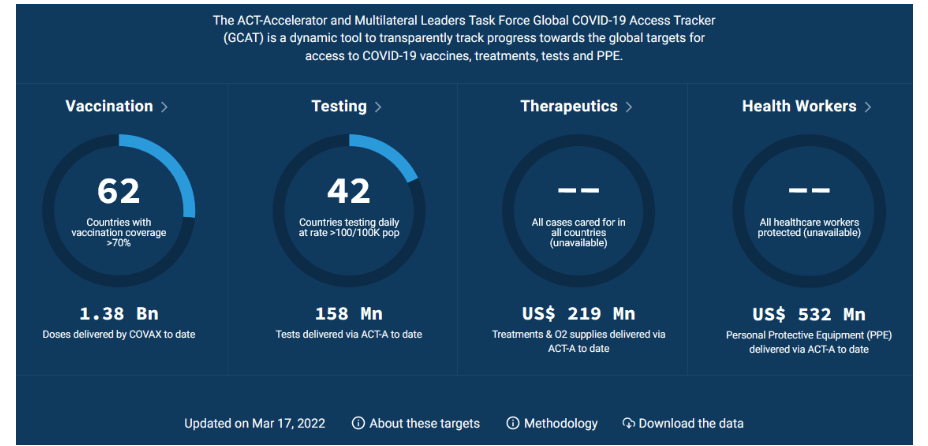
Pre-Print Servers
Gates Open Research
https://gatesopenresearch.org/browse/articles
Open Letter metrics AWAITING PEER REVIEW
Implementing adaptive youth-centered adolescent sexual reproductive health programming: learning from the Adolescents 360 project in Tanzania, Ethiopia, and Nigeria (2016-2020) [version 1; peer review: awaiting peer review]
Matthew Wilson, Meghan Cutherell, Abednego Musau, Sara Malakoff, Alexis Coppola, Metsehate Ayenekulu, Edwin Mtei, Fifi Ogbondeminu
Peer Reviewers Invited
Funders
Bill and Melinda Gates Foundation
Children’s Investment Fund Foundation
PUBLISHED 25 Mar 2022
medRxiv
https://www.medrxiv.org/content/about-medrxiv
medRxiv is a free online archive and distribution server for complete but unpublished manuscripts (preprints) in the medical, clinical, and related health sciences. Preprints are preliminary reports of work that have not been certified by peer review. They should not be relied on to guide clinical practice or health-related behavior and should not be reported in news media as established information. medRxiv is for the distribution of preprints – complete but unpublished manuscripts – that describe human health research conducted, analyzed, and interpreted according to scientific principles…
A Qualitative Study Regarding Messages of the COVID-19 Vaccine from Vaccinated Healthcare Providers and Healthy Adults
Shuji Sano, Satomi Sato, Norio Ohmagari, Osamu Takahashi
medRxiv 2022.03.24.22272878; doi: https://doi.org/10.1101/2022.03.24.22272878
International comparison of the impact of the pandemic and vaccination measures adopted on children and adolescent population
Luis Rajmil, Maria Camila Pinzon-Segura, Seth Christopher Yaw Appiah, Bernadine Ekpenyong, Fernando Gonzalez, Zuhal Gundogdu, Selim Oncel, Ozlem Cakici
medRxiv 2022.03.24.22272863; doi: https://doi.org/10.1101/2022.03.24.22272863
Booster dose of BNT162b2 in a CoronaVac primary vaccination protocol improves neutralization of SARS-CoV-2 Omicron variant
Guilherme R. F. Campos, Nathalie B. F. Almeida, Priscilla S. Filgueiras, Camila A. Corsini, Sarah V. C. Gomes, Daniel A. P. de Miranda, Jessica V. de Assis, Thais Barbara S. Silva, Pedro A Alves, Gabriel R. Fernandes, Jaquelline G. de Oliveira, Paula Rahal, Rafaella F. Q. Grenfell, Mauricio L Nogueira
medRxiv 2022.03.24.22272904; doi: https://doi.org/10.1101/2022.03.24.22272904
Impact assessment of mobility restriction, testing, and vaccination on the COVID-19 pandemic in India
Jeonghyun Shin, Quynh Long Khuong, Kaja Abbas, Juhwan Oh
medRxiv 2022.03.24.22272864; doi: https://doi.org/10.1101/2022.03.24.22272864
Vaccine Effectiveness Against Hospitalization Among Adolescent and Pediatric SARS-CoV-2 Cases in Ontario, Canada
Alison E. Simmons, Afia Amoako, Alicia Grima, Kiera Murison, Ashleigh Tuite, David Fisman
medRxiv 2022.03.24.22272919; doi: https://doi.org/10.1101/2022.03.24.22272919
Vaccine Adverse Event Reporting System (VAERS): Evaluation of 31 Years of Reports and Pandemics’ Impact
Ohoud Almadani, Thamir M Alshammari
medRxiv 2022.03.23.22272819; doi: https://doi.org/10.1101/2022.03.23.22272819
Systematic review and meta-analysis of yellow fever vaccine in elderly population
Ariane de Jesus Lopes de Abreu, João Roberto Cavalcante, Letícia Wigg de Araújo Lagos, Rosângela Caetano, José Ueleres Braga
medRxiv 2022.03.23.22272849; doi: https://doi.org/10.1101/2022.03.23.22272849
Vaccine effectiveness of two and three doses of BNT162b2 and CoronaVac against COVID-19 in Hong Kong
Martina E. McMenamin, Joshua Nealon, Yun Lin, Jessica Y. Wong, Justin K. Cheung, Eric H. Y. Lau, Peng Wu, Gabriel M. Leung, Benjamin J. Cowling
medRxiv 2022.03.22.22272769; doi: https://doi.org/10.1101/2022.03.22.2227276
Vaccine Equity in Low and Middle Income Countries: A Systematic Review and Meta-analysis
Huda Ali, Anna-Maria Hartner, Susy Echeverria-Londono, Jeremy Roth, Xiang Li, Kaja Abbas, Allison Portnoy, Emilia Vynnycky, Kim Woodruff, Neil M Ferguson, Jaspreet Toor, Katy AM Gaythorpe
medRxiv 2022.03.23.22272812; doi: https://doi.org/10.1101/2022.03.23.22272812
The World Health Organization’s Disease Outbreak News: a retrospective database
Colin J. Carlson, Matthew R. Boyce, Margaret Dunne, Ellie Graeden, Jessica Lin, Yasser Omar Abdellatif, Max A. Palys, Munir Pavez, Alexandra L. Phelan, Rebecca Katz
medRxiv 2022.03.22.22272790; doi: https://doi.org/10.1101/2022.03.22.22272790
People with HIV receiving suppressive antiretroviral therapy show typical antibody durability after dual COVID-19 vaccination, and strong third dose responses
Hope R. Lapointe, Francis Mwimanzi, Peter K. Cheung, Yurou Sang, Fatima Yaseen, Gisele Umviligihozo, Rebecca Kalikawe, Sarah Speckmaier, Nadia Moran-Garcia, Sneha Datwani, Maggie C. Duncan, Olga Agafitei, Siobhan Ennis, Landon Young, Hesham Ali, Bruce Ganase, F. Harrison Omondi, Winnie Dong, Junine Toy, Paul Sereda, Laura Burns, Cecilia T. Costiniuk, Curtis Cooper, Aslam H. Anis, Victor Leung, Daniel Holmes, Mari L. DeMarco, Janet Simons, Malcolm Hedgcock, Natalie Prystajecky, Christopher F. Lowe, Ralph Pantophlet, Marc G. Romney, Rolando Barrios, Silvia Guillemi, Chanson J. Brumme, Julio S.G. Montaner, Mark Hull, Marianne Harris, Masahiro Niikura, Mark A. Brockman, Zabrina L. Brumme
medRxiv 2022.03.22.22272793; doi: https://doi.org/10.1101/2022.03.22.22272793
“Where the truth really lies”: Listening to voices from African American communities in the Southern States about COVID-19 vaccine information and communication
Ran Zhang, Shan Qiao, Brooke McKeever, Bankole Olatosi, Xiaoming Li
medRxiv 2022.03.21.22272728; doi: https://doi.org/10.1101/2022.03.21.22272728
A Systematic Review of Human Challenge Trials, Designs, and Safety
Jupiter Adams-Phipps, Danny Toomey, Witold Więcek, Virginia Schmit, James Wilkinson, Keller Scholl, Euzebiusz Jamrozik, Joshua Osowicki, Meta Roestenberg, David Manheim
medRxiv 2022.03.20.22272658; doi: https://doi.org/10.1101/2022.03.20.22272658
Wellcome Open Research [to 26 Mar 2022]
https://wellcomeopenresearch.org/browse/articles
[Accessed 26 Mar 2022]
Wellcome Open Research provides all Wellcome researchers with a place to rapidly publish any results they think are worth sharing. All articles benefit from rapid publication, transparent peer review and editorial guidance on making all source data openly available.
Study Protocol metrics AWAITING PEER REVIEW
Yoga to improve maternal mental health and immune function during the COVID-19 crisis (Yoga-M2 trial): study protocol for a pilot randomized controlled trial. [version 1; peer review: awaiting peer review]
Rahul Shidhaye, Vidyadhar Bangal, Hemant Bhargav, Swanand Tilekar, Chitra Thanage, Rakhee Suradkar, Kalpesh Game, Vandana Pulate, Sonali Tambe, Vaibhav Murhar, Rahul Kunkulol
Peer Reviewers Invited
Funders
SATYAM (Science and Technology for Yoga and Meditation) Division, Department of Science and Technology, Government of India.
The Wellcome Trust DBT India Alliance
PUBLISHED 23 Mar 2022
Systematic Review metrics
Update
A living mapping review for COVID-19 funded research projects: one year update [version 5; peer review: 2 approved]
Alice Norton, Adrian Bucher, Emilia Antonio, Nicole Advani, Cathryn Johnston, Henrike Grund, Sheila Mburu, Emma Clegg, Marguerite Gollish, Sara Sahota, Nusrat Jabin, Laura Scott, Genevieve Boily-Larouche, A. Morgan Lay, Gail Carson, Marta Tufet Bayona
Peer Reviewers David Vaughn; Peter Smith
Funders
Wellcome Trust
European Commission
UK Research and Innovation
Department of Health, Social Services and Public Safety, UK Government
LATEST VERSION PUBLISHED 22 Mar 2022
Research Article metrics
Promotion of data sharing needs more than an emergency: An analysis of trends across clinical trials registered on the International Clinical Trials Registry Platform [version 1; peer review: 1 approved]
Laura Merson, Duduzile Ndwandwe, Thobile Malinga, Giuseppe Paparella, Kwame Oneil, Ghassan Karam, Robert F. Terry
Peer Reviewer Sharon Kaur Gurmukh Singh
Funders
Wellcome Trust
Special Programme for Research and Training in Tropical Diseases (TDR) and the World Heath Organisation
PUBLISHED 21 Mar 2022