The Lancet
Mar 12, 2022 Volume 399 Number 10329 p1023-1092, e12-e14
https://www.thelancet.com/journals/lancet/issue/current
Editorial
Ukraine’s humanitarian disaster: priorities for health
The Lancet
The Lancet
Mar 12, 2022 Volume 399 Number 10329 p1023-1092, e12-e14
https://www.thelancet.com/journals/lancet/issue/current
Editorial
Ukraine’s humanitarian disaster: priorities for health
The Lancet
Original Articles
Effectiveness of Covid-19 Vaccines over a 9-Month Period in North Carolina D.-Y. Lin and Others
New England Journal of Medicine
March 10, 2022 Vol. 386 No. 10
https://www.nejm.org/toc/nejm/medical-journal
Original Articles
Effectiveness of Covid-19 Vaccines over a 9-Month Period in North Carolina D.-Y. Lin and Others
New England Journal of Medicine
March 10, 2022 Vol. 386 No. 10
https://www.nejm.org/toc/nejm/medical-journal
New England Journal of Medicine
March 10, 2022 Vol. 386 No. 10
https://www.nejm.org/toc/nejm/medical-journal
njp Vaccines
https://www.nature.com/npjvaccines/
[Accessed 12 Mar 2022]
Brief Communication
08 Mar 2022
Three doses of BNT162b2 vaccine confer neutralising antibody capacity against the SARS-CoV-2 Omicron variant
Kevin K. Ariën, Leo Heyndrickx, Isabelle Desombere
PLoS Medicine
http://www.plosmedicine.org/
(Accessed 12 Mar 2022)
National adaptation and implementation of WHO Model List of Essential Medicines: A qualitative evidence synthesis
Elizabeth F. Peacocke, Sonja L. Myhre, Hakan Safaralilo Foss, Unni Gopinathan
Research Article | published 11 Mar 2022 PLOS Medicine
https://doi.org/10.1371/journal.pmed.1003944
PLoS One
http://www.plosone.org/
[Accessed 12 Mar 2022]
Research Article
Impact of catch-up human papillomavirus vaccination on cervical conization rate in a real-life population in France
Antoine Eliès, Claire Bonneau, Sophie Houzard, Roman Rouzier, Delphine Héquet
Research Article | published 11 Mar 2022 PLOS ONE
https://doi.org/10.1371/journal.pone.0264821
PLoS One
http://www.plosone.org/
[Accessed 12 Mar 2022]
Attitude of health professionals towards COVID-19 vaccination and associated factors among health professionals, Western Ethiopia: A cross-sectional survey
Tadesse Tolossa, Bizuneh Wakuma, Ebisa Turi, Diriba Mulisa, Diriba Ayala, Getahun Fetensa, Belayneh Mengist, Gebeyehu Abera, Emiru Merdassa Atomssa, Dejene Seyoum, Tesfaye Shibiru, Ayantu Getahun
Research Article | published 09 Mar 2022 PLOS ONE
https://doi.org/10.1371/journal.pone.0265061
PLoS One
http://www.plosone.org/
[Accessed 12 Mar 2022]
Reasons for COVID-19 vaccine refusal among people incarcerated in Canadian federal prisons
David Ortiz-Paredes, Olivia Varsaneux, James Worthington, Hyejin Park, Shannon E. MacDonald, Nicole E. Basta, Bertrand Lebouché, Joseph Cox, Shainoor J. Ismail, Nadine Kronfli
Research Article | published 09 Mar 2022 PLOS ONE
https://doi.org/10.1371/journal.pone.0264145
PNAS – Proceedings of the National Academy of Sciences of the United States
February 15, 2022 | vol. 119 | no. 7
https://www.pnas.org/toc/pnas/119/7
Research Article February 1, 2022 Open Access
Real-time pandemic surveillance using hospital admissions and mobility data
Forecasting the burden of COVID-19 has been impeded by limitations in data, with case reporting biased by testing practices, death counts lagging far behind infections, and hospital census reflecting time-varying patient access, admission criteria, and …
Spencer J. Fox, Michael Lachmann,[…] Lauren Ancel Meyers
Preventive Medicine
Volume 156 March 2022
https://www.sciencedirect.com/journal/preventive-medicine/vol/156/suppl/C
Discussion Full text access
HPV prevention and control – The way forward
Alex Vorsters, F. Xavier Bosch, Mario Poljak, Dur-e-Nayab Waheed, … Suzanne M. Garland
Article 106960
Preventive Medicine
Volume 156 March 2022
https://www.sciencedirect.com/journal/preventive-medicine/vol/156/suppl/C
Regular Articles
Research article Full text access
Intention to vaccinate children for COVID-19: A segmentation analysis among Medicaid parents in Florida
Matthew W. Kreuter, Rachel Garg, Alexis Marsh, Tess Thompson, … Amy McQueen
Article 106959
Public Health
Volume 204 Pages 1-86 (March 2022)
https://www.sciencedirect.com/journal/public-health/vol/204/suppl/C
Research article Abstract only
Factors associated with incomplete child immunization in Pakistan: findings from Demographic and Health Survey 2017-18
A. Ali, A. Zar, A. Wadood
Pages 43-48
Risk Management and Healthcare Policy
https://www.dovepress.com/risk-management-and-healthcare-policy-archive56
[Accessed 12 Mar 2022]
Original Research
Motors of COVID-19 Vaccination Acceptance Scale (MoVac-COVID19S): Evidence of Measurement Invariance Across Five Countries
Chen IH, Wu PL, Yen CF, Ullah I, Shoib S, Zahid SU, Bashir A, Iqbal N, Addo FM, Adjaottor ES, Amankwaah GB, Ahorsu DK, Griffiths MD, Lin CY, Pakpour AH
Published Date: 10 March 2022
Science
Volume 375| Issue 6585| 11 Mar 2022
https://www.science.org/toc/science/current
Special issue: COVID-19: 2 years on
Introduction to Special Issue
A time to reflect
BY Caroline Ash. Et al.
10 Mar 2022: 1098-1099
Free
Science
Volume 375| Issue 6585| 11 Mar 2022
https://www.science.org/toc/science/current
Editorial
“Back to normal” is not enough
BY Christina Pagel
10 Mar 2022: 1069-1069
Science
Volume 375| Issue 6585| 11 Mar 2022
https://www.science.org/toc/science/current
Policy Forum
End COVID-19 in low- and middle-income countries
BY Ahmed Mushfiq Mobarak et al.
10 Mar 2022: 1105-1110
Free
Science
Volume 375| Issue 6585| 11 Mar 2022
https://www.science.org/toc/science/current
Gender-responsive social protection post–COVID-19
BY Maja Gavrilovic, et al.
10 Mar 2022: 1111-1113
Free
Investment in gender-responsive social protection systems and evidence is key to a more equal future post–COVID-19
Science
Volume 375| Issue 6585| 11 Mar 2022
https://www.science.org/toc/science/current
Reviews
COVID-19 vaccination: The road ahead
BY Daniel M. Altmann, Rosemary J. Boyton
10 Mar 2022: 1127-11
A diverse array of successful, first-generation SARS-CoV-2 vaccines have played a huge role in efforts to bring the COVID-19 pandemic under control, even though inequitable distribution still leaves many vulnerable. Additional challenges loom for the next …
Science
Volume 375| Issue 6585| 11 Mar 2022
https://www.science.org/toc/science/current
Reports
Vaccination with BNT162b2 reduces transmission of SARS-CoV-2 to household contacts in Israel
BY Ottavia Prunas. Et al.
27 Jan 2022: 1151-1154
Open Access
Vaccination reduced both the rate of infection with SARS-CoV-2 and household transmission in Israel.
Science
Volume 375| Issue 6585| 11 Mar 2022
https://www.science.org/toc/science/current
Indirect protection of children from SARS-CoV-2 infection through parental vaccination
BY Samah Hayek et al.
27 Jan 2022: 1155-1159
Open Access
Parental Covid-19 vaccination confers substantial protection for unvaccinated children in the household.
Tropical Medicine & International Health
Volume 27, Issue 3 Pages: i-iv, 217-336 March 2022
https://onlinelibrary.wiley.com/toc/13653156/current
RESEARCH ARTICLES
Impact of meningococcal C conjugate vaccine on incidence of invasive meningococcal disease in an 18-year time series in Brazil and in distinct Brazilian regions
Mariana C. Cruz, Paulo Camargos, Cristiana M. Nascimento-Carvalho
Pages: 280-289
First Published: 08 January 2022
Vaccine
Volume 40, Issue 8 Pages 1061-1190 (16 February 2022)
https://www.sciencedirect.com/journal/vaccine/vol/40/issue/8
Research article Full text access
Priming with social benefit information of vaccination to increase acceptance of COVID-19 vaccines
Qiuyan Liao, Benjamin J. Cowling, Jingyi Xiao, Jiehu Yuan, … Wendy Wing Tak Lam
Pages 1074-1081
Vaccine
Volume 40, Issue 8 Pages 1061-1190 (16 February 2022)
https://www.sciencedirect.com/journal/vaccine/vol/40/issue/8
Research article Open access
‘I trust them because my mum trusts them’: Exploring the role of trust in HPV vaccination decision-making among adolescent girls and their mothers in France
E. Karafillakis, P. Peretti-Watel, P. Verger, T. Chantler, H.J. Larson
Pages 1090-1097
Vaccine
Volume 40, Issue 8 Pages 1061-1190 (16 February 2022)
https://www.sciencedirect.com/journal/vaccine/vol/40/issue/8
Research article Abstract only
Factors associated with maternal tetanus vaccination in Myanmar: An analysis of demographic and health survey data
Zaw Myo Tun, Zau Ring, Clarence C. Tam
Pages 1135-1142
Vaccine
Volume 40, Issue 8 Pages 1061-1190 (16 February 2022)
https://www.sciencedirect.com/journal/vaccine/vol/40/issue/8
Research article Open access
COVID-19 vaccine uptake, effectiveness, and waning in 82,959 health care workers: A national prospective cohort study in Wales
Stuart Bedston, Ashley Akbari, Christopher I. Jarvis, Emily Lowthian, … Ronan A. Lyons
Pages 1180-1189
Pre-Print Servers
Gates Open Research
https://gatesopenresearch.org/browse/articles
[Accessed 12 Mar 2022]
[No new digest content identified]
medRxiv
https://www.medrxiv.org/content/about-medrxiv
medRxiv is a free online archive and distribution server for complete but unpublished manuscripts (preprints) in the medical, clinical, and related health sciences. Preprints are preliminary reports of work that have not been certified by peer review. They should not be relied on to guide clinical practice or health-related behavior and should not be reported in news media as established information. medRxiv is for the distribution of preprints – complete but unpublished manuscripts – that describe human health research conducted, analyzed, and interpreted according to scientific principles…
Immunogenicity and safety of a recombinant adenovirus type-5 COVID 19 vaccine in adults: data from a randomised, double-blind, placebo-controlled, single-dose, phase 3 trial in Russia
Vitalina Dzutseva, Dmitry Lioznov, Irina Amosova, Savely A Sheetikov, Ksenia V Zornikova, Yana Serdyuk, Grigory A Efimov, Mikhail Tsyferov, Mikhail Khmelevskii, Andrei Afansiev, Nadezdha Khomyakova, Dmitry Zubkov, Anton Tikhonov, Tao Zhu, Luis Barreto
medRxiv 2022.03.01.22271507; doi: https://doi.org/10.1101/2022.03.01.22271507
Real world performance of inactivated SARS-CoV-2 vaccine (CoronaVac) against infection, hospitalization and death due to COVID-19 in adult population in Indonesia
Anton Suryatma, Raras Anasi, Miko Hananto, Asep Hermawan, Ririn Ramadhany, Irene Lorinda Indalao, Agustiningsih Agustiningsih, Ely Hujjatul Fikriyah, Kristina Lumban Tobing, Teti Tejayanti, Rustika Rustika, Pandji Wibawa Dhewantara
medRxiv 2022.02.02.22270351; doi: https://doi.org/10.1101/2022.02.02.22270351
Impact of SARS-CoV-2 vaccination of children ages 5-11 years on COVID-19 disease burden and resilience to new variants in the United States, November 2021-March 2022: a multi-model study
Rebecca K. Borchering, Luke C. Mullany, Emily Howerton, Matteo Chinazzi, Claire P. Smith, Michelle Qin, Nicholas G. Reich, Lucie Contamin, John Levander, Jessica Kerr, J Espino, Harry Hochheiser, Kaitlin Rainwater-Lovett, Matt Kinsey, Kate Tallaksen, Shelby Wilson, Lauren Shin, Joseph C. Lemaitre, Juan Dent Hulse, Joshua Kaminsky, Elizabeth C. Lee, Javier Perez-Saez, Jessica T. Davis, Kunpeng Mu, Xinyue Xiong, Ana Pastore y Piontti, Alessandro Vespignani, Ajitesh Srivastava, Przemyslaw Porebski, Srinivasan Venkatramanan, Aniruddha Adiga, Bryan Lewis, Brian Klahn, Joseph Outten, Benjamin Hurt, Jiangzhuo Chen, Henning Mortveit, Amanda Wilson, Madhav Marathe, Stefan Hoops, Parantapa Bhattacharya, Dustin Machi, Shi Chen, Rajib Paul, Daniel Janies, Jean-Claude Thill, Marta Galanti, Teresa K. Yamana, Sen Pei, Jeffrey Shaman, Guido España, Sean Cavany, Sean Moore, Alex Perkins, Jessica M. Healy, Rachel B. Slayton, Michael A. Johansson, Matthew Biggerstaff, Katriona Shea, Shaun Truelove, Michael C. Runge, Cécile Viboud, Justin Lessler
medRxiv 2022.03.08.22271905; doi: https://doi.org/10.1101/2022.03.08.22271905
Community Engagement to support COVID-19 Vaccine Uptake: A living systematic review protocol
Brynne Gilmore, Nina Gerlach, Claudia Abreu Lopes, Alpha Ahmadou Diallo, Sanghita Bhattacharyya, Vergil de Claro, Rawlance Ndejjo, Elizabeth Nyamupachitu- Mago, Adalbert Tchetchia
medRxiv 2022.03.08.22272082; doi: https://doi.org/10.1101/2022.03.08.22272082
Reasons underlying the intention to vaccinate children aged 5-11 against COVID-19: A cross-sectional study of parents in Israel, November 2021
Nicole G. Morozov, Amiel A. Dror, Amani Daoud, Netanel Eisenbach, Edward Kaykov, Masad Barhoum, Tsvi Sheleg, Eyal Sela, Michael Edelstein
medRxiv 2022.03.03.22271793; doi: https://doi.org/10.1101/2022.03.03.22271793
Social capital, urbanization level, and COVID-19 vaccination uptake in the United States: A national level analysis
Shan Qiao, Zhenlong Li, Jiajia Zhang, Xiaowen Sun, Camryn Garrett, Xiaoming Li
medRxiv 2022.03.04.22271917; doi: https://doi.org/10.1101/2022.03.04.22271917
Responsiveness to risk explains large variation in COVID-19 mortality across countries
Tse Yang Lim, Hazhir Rahmandad
medRxiv 2020.12.11.20247924; doi: https://doi.org/10.1101/2020.12.11.20247924
Using Google Health Trends to investigate COVID-19 incidence in Africa
Alexander Fulk, Daniel Romero-Alvarez, Qays Abu-Saymeh, Jarron M. Saint Onge, A. Townsend Peterson, Folashade B. Agusto
medRxiv 2021.03.26.21254369; doi: https://doi.org/10.1101/2021.03.26.21254369
Wellcome Open Research [to 12 Mar 2022]
https://wellcomeopenresearch.org/browse/articles
[Accessed 12 Mar 2022]
Wellcome Open Research provides all Wellcome researchers with a place to rapidly publish any results they think are worth sharing. All articles benefit from rapid publication, transparent peer review and editorial guidance on making all source data openly available.
Open Letter metrics AWAITING PEER REVIEW
A PowerPack of SuperScientists: An innovative concept by African scientists to address gender bias and inequity in science [version 1; peer review: awaiting peer review]
Maphe Mthembu, Omolara Baiyegunhi, Yanga Mdleleni, Lerato Ndlovu, Hannah Keal, Kim Waddilove, Justin C. Yarrow, Victoria Kasprowicz, Thumbi Ndung’u, Emily B. Wong
Peer Reviewers Invited
Funders
African Academy of Sciences
New Partnership for Africa’s Development
Government of the United Kingdom
Wellcome
PUBLISHED 11 Mar 2022
Research Article metrics
Revised
Risk factors for SARS-CoV-2 seroprevalence following the first pandemic wave in UK healthcare workers in a large NHS Foundation Trust [version 2; peer review: 1 approved with reservations]
Hayley Colton, David Hodgson, Hailey Hornsby, Rebecca Brown, Joanne Mckenzie, Kirsty L. Bradley, Cameron James, Benjamin B. Lindsey, Sarah Birch, Louise Marsh, Steven Wood, Martin Bayley, Gary Dickson, David C. James, Martin J. Nicklin, Jon R. Sayers, Domen Zafred, Sarah L. Rowland-Jones, Goura Kudesia, Adam Kucharski, CMMID COVID-19 Working Group, Thomas C. Darton, Thushan I. de Silva, Paul J. Collini
Peer Reviewer Mo Yin
Funders
Wellcome Trust
The Danson Foundation
Sheffield Teaching Hospitals NHS Foundation Trust
LATEST VERSION PUBLISHED 10 Mar 2022
Review metrics AWAITING PEER REVIEW
Innovations in peer review in scholarly publishing: a meta-summary [version 1; peer review: awaiting peer review]
Helen Buckley Woods, Johanna Brumberg, Wolfgang Kaltenbrunner, Stephen Pinfield, Ludo Waltman
Peer Reviewers Invited
Funder
Wellcome Trust
PUBLISHED 09 Mar 2022
Research Article metrics AWAITING PEER REVIEW
Quantitative methods for group bibliotherapy research: a pilot study [version 1; peer review: awaiting peer review]
Emily T. Troscianko, Emily Holman, James Carney
Peer Reviewers Invited
Funders
Wellcome Trust
Balliol Interdisciplinary Institute
PUBLISHED 07 Mar 2022
Think Tanks
Brookings
http://www.brookings.edu/
Accessed 12 Mar 2022
Order from Chaos
Taiwan’s people are not impressed with China’s “Zero COVID” status
Shelley Rigger, Lev Nachman, Chit Wai John Mok, and Nathan Kar Ming Chan
Friday, March 11, 2022
TechStream
China and Russia are joining forces to spread disinformation
David Bandurski
Friday, March 11, 2022
Center for Global Development [to 12 Mar 2022]
https://www.cgdev.org/
Accessed 12 Mar 2022
Last Week in the US International COVID-19 Response
March 8, 2022
In the global response to COVID-19, the Biden administration made news last week on several fronts. Here is a quick recap and some ideas for moving forward.
Jocilyn Estes, Julia Kaufman and Eleni Smitham
Chatham House [to 12 Mar 2022]
https://www.chathamhouse.org/
Accessed 12 Mar 2022
[No new digest content identified]
CSIS
https://www.csis.org/
Accessed 12 Mar 2022
Podcast Episode
Dr. Heidi Larson: “The nature of the security threat has changed”
March 11, 2022 | By Katherine E. Bliss, Heidi J. Larson
Transcript
Overcoming Gender-Related Barriers to Immunization Services
March 10, 2022
Report
North Korea’s Covid-19 Lockdown: Current Status and Road Ahead
March 9, 2022 | By Victor Cha, Katrin Fraser Katz, J. Stephen Morrison
Kaiser Family Foundation
https://www.kff.org/search/?post_type=press-release
Accessed 12 Mar 2022
March 10, 2022 News Release
4 in 10 Workers – and 6 in 10 of Those with Low Incomes – Say They Missed Work During the Omicron Surge Due to COVID-19 Illness, Quarantine or Closure
The surge in COVID-19 cases triggered by the omicron variant led to widespread work disruptions, with about 4 in 10 workers (42%) – including 6 in 10 of those with lower incomes – saying they had to miss work at least once in the past three months because of a…
Rand [to 12 Mar 2022]
https://www.rand.org/pubs.html
Reports, Selected Journal Articles
[No new digest content identified]
Vaccines and Global Health: The Week in Review is a weekly digest summarizing news, events, announcements, peer-reviewed articles and research in the global vaccine ethics and policy space. Content is aggregated from key governmental, NGO, international organization and industry sources, key peer-reviewed journals, and other media channels. This summary proceeds from the broad base of themes and issues monitored by the Center for Vaccine Ethics & Policy in its work: it is not intended to be exhaustive in its coverage. You are viewing the blog version of our weekly digest, typically comprised of between 30 and 40 posts below all dated with the current issue date
.– Request an Email Summary: Vaccines and Global Health : The Week in Review is published as a single email summary, scheduled for release each Saturday evening before midnight (EDT in the U.S.). If you would like to receive the email version, please send your request to david.r.curry@centerforvaccineethicsandpolicy.org.
– pdf version: A pdf of the current issue is available here:
– blog edition: comprised of the approx. 35+ entries posted below.
– Twitter: Readers can also follow developments on twitter: @vaxethicspolicy.
.
– Links: We endeavor to test each link as we incorporate it into any post, but recognize that some links may become “stale” as publications and websites reorganize content over time. We apologize in advance for any links that may not be operative. We believe the contextual information in a given post should allow retrieval, but please contact us as above for assistance if necessary.
Support this knowledge-sharing service: Your financial support helps us cover our costs and to address a current shortfall in our annual operating budget. Click here to donate and thank you in advance for your contribution.
.
David R. Curry, MS
Executive Director
Center for Vaccine Ethics and Policy
Ukraine
WHO – Ukraine Emergency
WHO is working closely with our offices in Ukraine and neighbouring countries, as well as partners to rapidly respond to the health emergency triggered by the conflict and to minimize disruptions to the delivery of critical healthcare services.
WHO continues to deliver much-needed support on urgent health needs. During the crisis, health must remain a priority pillar, with health workers being protected so they can continue to save lives and with health systems and facilities being protected so that they remain functional, safe and accessible to all who need essential medical services. It is imperative to ensure that life-saving medical supplies – including oxygen – reach those who need them.
Emergency in Ukraine – Situation Report 1
5 March 2022 | Emergency Situational Updates
[Excerpts]

Priority public health concerns
Conflict related trauma and injuries exacerbated by lack of access to health facilities by patients and health staff due to insecurity and lack of access to lifesaving medicine and supplies.
Excess morbidity and death from common illnesses due to disruption in services such as non-communicable diseases (cardiovascular, diabetes, cancer etc.) and acute maternal, newborn and child illnesses.
Spread of infectious diseases such as COVID-19, measles, polio, TB, HIV and diarrheal diseases due to widespread destruction of water and sanitation infrastructure, inadequate vaccination coverage, lack of access to medicines and medical care, safe water, adequate sanitation and hygiene as well as population movements and crowding.
Mental health and psychosocial health – due to significant stress due to acute conflict and two years of COVID-19.
…2.3. Epidemic prone and other infectious diseases
Recent outbreaks of polio and measles threaten the health of populations with suboptimal vaccination coverage (80% and 82% respectively in 2021), and the prevalence of HIV and tuberculosis, including multidrug resistant tuberculosis, are among the highest in Europe. Urgent actions are to re-start or continue preventive measures through vaccination and continued treatment for TB and HIV and to scale up surveillance, early detection and response systems for epidemic-prone diseases.
A polio outbreak (circulating vaccine-derived poliovirus type 2) was confirmed in the country in 2021, with two paralytic cases (detected in October and December 2021), and a total of 21 individuals in two oblasts (Rivne and Zakarpattya) who had positive isolation of cVDPV2 in stool specimens. As a result, a nationwide vaccination campaign targeting all under-vaccinated children (those having only zero or one dose) aged between 6 months and 6 years that began in February 2022. This campaign has been suspended because of the conflict, increasing the risk of further spread.
Over 240 000 weekly cases and 1300 deaths were reported for COVID-19 in the week 21-27 February. While this is a 43% decrease in cases compared to the previous week, testing rates have also declined sharply since the start of the conflict, with a likely significant undetected ongoing transmission. The ongoing high incidence levels of COVID-19 poses a significant risk of severe disease and death, particularly given the low vaccination coverage in at-risk population groups. Critical shortages of oxygen further impact on the ability to treat patients with severe COVID-19, and many other conditions. Beds occupied by COVID-19 patients were repurposed for trauma injuries and critical illnesses.
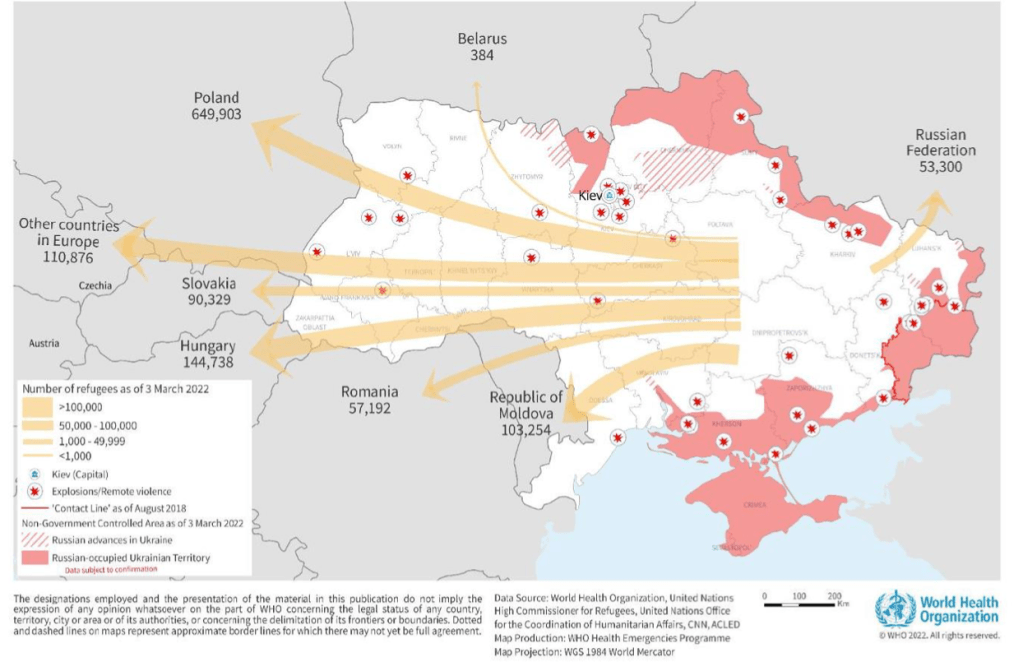
Emergency appeal – Ukraine and neighbouring countries
2 March 2022
U.S. Treasury Prohibits Transactions with Central Bank of Russia and Imposes Sanctions on Key Sources of Russia’s Wealth
Press Release
February 28, 2022
In Coordination with Allies and Partners, Treasury Actions Restrict Access to Billions in Central Bank Assets and Sovereign Wealth Fund
Treasury Sanctions Russian Direct Investment Fund – A Symbol of Russian Kleptocracy
United States and its Partners and Allies Continue to Hold the Government of the Russian Federation Accountable for its Premeditated and Unprovoked Invasion of Ukraine
::::::
RDIF statement
Press release, 28.02.2022
The Russian Direct Investment Fund (RDIF, the sovereign wealth fund of the Russian Federation) was never involved in any political activities, does not interact in any way with Ukraine and follows the world’s best investment practices, which has been acknowledged by all its international partners as well by national regulators. RDIF always fully complies with laws of the countries where it conducts its investments.
Imposition of sanctions against RDIF, which from the moment it was established has stood for building international relations and supporting constructive ties, demonstrates that the U.S. has picked the course to destroy constructive dialogue between countries.
In the past two years, the Fund has been focusing on ensuring global epidemiological safety through its pivotal involvement in struggle against the new coronavirus infection in over 70 countries. In particular, RDIF is the key investor in unique test systems to detect coronavirus infection, anti-COVID medicine as well as in Russian vaccines Sputnik V and Sputnik Light, saving millions of lives by supplying vaccines and facilitating their production in Russia and other countries.
The restrictions imposed by the U.S. authorities complicating RDIF efforts on the international promotion of the Russian vaccine products, have been lobbied by a number of large Western pharmaceutical companies. As a result of such unfair competition, billions of people around the world may be deprived of access to effective and safe Russian-made vaccines….
European Commission suspends cooperation with Russia on research and innovation
Press release 4 March 2022
Following the Russian invasion against Ukraine and in solidarity with the people of Ukraine, the Commission has decided to suspend the cooperation with Russian entities in research, science and innovation. The Commission will not conclude any new contracts nor any new agreements with Russian organisations under the Horizon Europe programme. Furthermore, the Commission is suspending payments to Russian entities under existing contracts. All ongoing projects, in which Russian research organisations are participating, are being reviewed – both under Horizon Europe and Horizon 2020, the previous EU programme for research and innovation.
Margrethe Vestager, Executive Vice-President for a Europe fit for the Digital Age, said: “EU research cooperation is based on the respect for the freedoms and rights that underpin excellence and innovation. Russia’s heinous military aggression against Ukraine is an attack against those same values. It is therefore time to put an end to our research cooperation with Russia.”…
… Ukrainian scientists and researchers are key participants in our EU Framework programmes for R&I and have demonstrated excellence and innovation leadership across many fields.
We are strongly committed to ensuring a continued successful participation of Ukraine and Ukrainian entities in Horizon Europe and Euratom Research and Training programmes…
The full statement of Commissioner Gabriel is available here. The assessment of the situation as regards Belarus is ongoing.
::::::
Malaria
First-ever malaria vaccine recommendation now published in a position paper and in the WHO guidelines for malaria
4 March 2022 Departmental news
WHO today published an updated position paper on the RTS,S/AS01 (RTS,S) malaria vaccine that includes the October 2021 recommendation calling for the wider use of the vaccine among children living in areas of moderate-to-high P. falciparum malaria transmission. The paper complements the recent addition of the recommendation to the WHO Guidelines for malaria.
“The first malaria vaccine is a major step forward for malaria control, child health and health equity. If implemented broadly, the vaccine could save tens of thousands of lives each year,” said Dr Kate O’Brien, director of the Department of Immunization, Vaccines and Biologicals. “This guidance is essential to countries as they consider whether and how to adopt the vaccine as an additional tool to reduce child illness and deaths from malaria,” she added.
…Coming soon: additional malaria vaccine tools for countries
This newly published guidance will be followed in the coming months by additional tools and information to guide countries that have decided to adopt the malaria vaccine – including a new malaria vaccine introduction guide and an operational manual for sub-national tailoring of malaria control tools…
Malaria vaccine: WHO position paper – March 2022
4 March 2022
Overview
This position paper supersedes the 2016 publication, “Malaria vaccine: WHO position paper-2016.”1 It includes the updated WHO recommendations on the wider use of the RTS,S/AS01 vaccine for the reduction of malaria morbidity and mortality in children living in areas of moderate to high malaria transmission. It also incorporates findings from the evaluation of the WHO-coordinated Malaria Vaccine Implementation Programme (MVIP), recommended by SAGE and MPAG in 2015, and from additional studies since 2015.
This paper does not include findings on vaccine efficacy in infants first vaccinated at 6–12 weeks of age. Because of the lower vaccine efficacy observed in this age category, WHO did not recommend pilot implementation or RTS,S/AS01 vaccine introduction for these young infants. Recommendations2 on the use of RTS,S/AS01 vaccine were discussed by SAGE and MPAG during a joint session in October 2021; evidence presented at the meeting can be accessed at https://terrance.who.int/mediacentre/data/ sage/SAGE_eYB_Oct2021.pdf
Workshop on WHO policy guidance on malaria elimination and the implementation of the intensification plans for reducing malaria burden in the Greater Mekong subregion
Virtual meeting, 22–23 November 2021
1 March 2022
Overview
The Workshop was convened virtually from 22 to 23 November 2021. Organized by the Mekong Malaria Elimination (MME) programme, it brought together participants from the 6 GMS countries – Cambodia, China, the Lao People’s Democratic Republic, Myanmar, Thailand and Viet Nam – and the Global Malaria Programme, as well as technical experts and partners to help GMS countries with the task of eliminating malaria and prepare them for the process of verification and certification of malaria
AMR
Reducing Antimicrobial Discharges from Food Systems, Manufacturing Facilities and Human Health Systems into the Environment
Call to Action by the Global Leaders Group on Antimicrobial Resistance
March 2022
Disposal of untreated or inappropriately managed waste and runoff from various sources including food systems, manufacturing facilities and human health systems can contain biologically active antimicrobials, antimicrobial resistant organisms, unmetabolized antimicrobials and antimicrobial resistance determinants (e.g. resistance-conferring genes) that are released into the environment.
These discharges can contaminate the environment and contribute to the spread of antimicrobial resistance (AMR). The most important approach to controlling AMR spread from food systems and human health systems is responsible and sustainable use of antimicrobials in humans, terrestrial and aquatic animals and plants/crops. In addition, adequate measures to treat and safely dispose of waste are required, including human, animal and manufacturing waste.
The GLG commends ongoing efforts – particularly by the G7 countries – to address antimicrobial discharges into the environment and encourages countries to implement the Codex Code of practice to minimize and contain foodborne AMR and Guidelines on Integrated Monitoring and Surveillance of Foodborne Antimicrobial Resistance approved in November 2021.
To improve the management of discharges into the environment that may contribute to the emergence and spread of antimicrobial resistance, the Global Leaders Group calls for the following:
1. STRENGTHENED GOVERNANCE AND OVERSIGHT
In general, countries should:
Develop or build on and implement regulatory frameworks, guidelines, standard operating procedures (SOPs) and standards to establish safe levels, better control and monitor the distribution and release of antimicrobials, antimicrobial resistant bacteria and antimicrobial resistance determinants from food systems, manufacturing facilities and human health systems into the environment; and
Include prevention and management measures in national action plans on AMR to minimize the impacts of environmental discharges.
In the manufacturing sector specifically, countries should:
Develop and implement legal and policy frameworks with a lifecycle approach for antimicrobials manufacturing. Such an approach considers the entire timespan that a pharmaceutical is active and can impact the surrounding systems, would help to effectively address AMR environmental risks and ensure resilient antibiotic supply chains and stimulate the design, development, manufacture, and commercialization of needed new antibiotics and alternatives to antimicrobials;
Promote and develop balanced and staged environmental policies and approaches to manage and regulate manufacturing facilities and support environmental inspections, recognizing the current fragility of supply chains and significant access gaps;
Incentivize industry for compliance and excellence, including highlighting their contribution to the achievement of the Sustainable Development Goals; and
Develop national antimicrobial manufacturing pollution standards based on best available evidence, treatment technology and situational analysis, and strengthen the capacity of environmental authorities to conduct audits and monitor compliance.
In the human health sector specifically, countries should:
Develop and implement antimicrobial stewardship policies and protocols in human health systems that include responsible and sustainable use and procurement of antimicrobials, and effective waste management approaches; and
Implement and enforce laws and policies to reduce or eliminate antimicrobial use that is not under the guidance of a trained health care provider, while ensuring equitable access to quality antimicrobials.
In food systems specifically, countries should:
Develop or build on and implement regulatory frameworks, guidelines, SOPs and standards to effectively treat and/or manage waste discharge from food-producing animal farms, aquaculture farms and crop fields, as well as waste used to irrigate crops and run-off from crop fields; and
Develop and implement antimicrobial stewardship policies and protocols in fixed and mobile animal health facilities that include responsible and sustainable use of antimicrobials and effective waste management approaches.
2. IMPROVED SURVEILLANCE AND DATA AVAILABILITY
Countries should:
Strengthen One Health surveillance of antimicrobial use in, and discharges of antimicrobials and AMR determinants from, food systems, human health systems and manufacturing facilities, as well as in sewage systems. This should be done taking into account factors such as the need to build on existing systems, cost-effectiveness, data comparability and key knowledge gaps relating to the fate, concentration and impact of discharges on the environment and organisms in the environment (e.g. soil microbes, aquatic organisms). Priority should be given to collecting data that can support targeted action, such as enhanced understanding of risks to human and animal health and the environment and release pathways into the environment, and supporting the development of guidance on waste management approaches and antimicrobial discharge limits; and
Promote industry data disclosure, transparency and public access to waste and wastewater management data and mitigation practices in order to build credibility and public confidence. Data disclosure could initially be made to regulators and independent third parties (for example as part of certification schemes), followed by efforts to enable wider public access to increase awareness and understanding, contribute to ongoing studies and reflect environmental standards in procurement practices.
3. IMPROVED DISCHARGE MANAGEMENT
In general, all countries should:
Reduce the need for antimicrobial use through implementation of effective infection prevention and control measures in all sectors, including water, sanitation and hygiene (WASH), vaccination, biosecurity and animal husbandry and welfare measures;
Develop, implement and monitor systems for proper segregation, treatment and/or disposal of antimicrobials and antimicrobial-containing substances in all sectors (including antimicrobial feed and human and animal waste);
Develop mechanisms for collection and proper disposal of unused and expired antimicrobials from individuals and organizations; and
Ensure availability of affordable and environmentally safe incinerators and innovative technologies for destruction and degradation of unused or expired antimicrobials.
In general, relevant international technical organizations and their partners should develop guidance and showcase best practices on proper waste management practices across sectors.
In food systems:
All countries should:
Create and implement manure, wastewater, runoff and farm waste management plans, SOPs, guidance, standards and measures such as composting for manure and its application into agriculture fields; and
Implement evidence-based manure management approaches so that manure can continue to be safely used as a natural fertilizer in agriculture fields and support agro-ecological farming practices while minimizing the risks of transfer of antimicrobial resistant bacteria or antimicrobial resistance determinants.
International technical organizations should:
Expedite the development of tools and guidance to support the implementation of the Codex Code of practice to minimize and contain foodborne AMR and Guidelines on Integrated Monitoring and Surveillance of Foodborne Antimicrobial Resistance along the food chain (e.g. food processing and production facilities, wet markets, slaughterhouses) to minimize the impacts of antimicrobial discharge into the environment.
Companies involved in the slaughter and processing of food animals should:
Assess current food production practices to implement measures to reduce discharges of by-products, including biocides, into the environment and comply with legal standards and requirements.
In the manufacturing sector:
Manufacturing companies should:
Commit to prevention and management measures to minimize the impacts of manufacturing discharges into the environment . This can be done through effective waste management technologies and practices, adoption and implementation of the common antibiotic manufacturing framework
and the proposed independent certification schemes of the AMR Industry Alliance.
All stakeholders should:
Evaluate options and support efforts to create an enabling environment that influences and supports investment through incentives and efforts in pharmaceutical waste management without jeopardizing access to antimicrobials. Such evaluations may include an assessment of sustainable procurement policies, inclusion of environmental considerations in good manufacturing practices, environmental risk assessment before antimicrobial authorization and an independent product-certification scheme.
4. RESEARCH AND DEVELOPMENT
International technical, financing and research and development organizations and partners should:
Enhance and coordinate research for a comprehensive understanding of risks to human and animal health from the environmental presence of antimicrobials, resistance microbes and mobile genetic elements in discharges, as well as potential hot spots, environmental impacts and antimicrobial resistance pathways, and mitigation measures;
Promote research and development across public and private sectors into cost-effective and greener waste management technologies including methods to remove antimicrobial residues, resistance genes and resistant organisms and other tools (e.g., climate-sensitive incinerators and measurement technologies) and standardized monitoring methods, and support mainstreaming of best practices in process and waste management across sectors; and
Develop policy briefs on antimicrobial resistance and organize policy dialogues among policymakers to support evidence-based policymaking.
Global Leaders Group on Antimicrobial Resistance background
The Global Leaders Group on Antimicrobial Resistance was established in November 2020 and performs an independent global advisory and advocacy role with the primary objective of maintaining urgency, public support, political momentum and visibility of the AMR challenge on the global agenda. The mission of the group is to collaborate globally with governments, agencies, civil society and the private sector through a One Health approach to advise on and advocate for political action for the mitigation of drug-resistant infections through responsible and sustainable access to and use of antimicrobials.
The group is co-chaired by Their Excellencies Sheikh Hasina, Prime Minister of Bangladesh and Mia Amor Mottley, Prime Minister of Barbados and is composed of heads of state, serving or former ministers and/or senior government officials acting in their individual capacities, together with senior representatives of foundations, civil society organizations and the private sector. It also includes principals of the Tripartite organizations – the Food and Agriculture Organization of the United Nations (FAO), the World Organisation for Animal Health (OIE) and the World Health Organization (WHO), and the UN Environment Programme (UNEP) – in an ex-officio capacity. The Tripartite Joint Secretariat (TJS), a joint effort by FAO, OIE and WHO, and UNEP, provides Secretariat support for the Group.
COVID – ACT-Accelerator
ACT-Accelerator welcomes Germany’s generous ‘fair share’ commitment
1 March 2022
Statement
Germany has become the first country to pledge to meet its ‘fair share’ of the ACT-Accelerator’s 2021/22 budget, with a generous contribution of US$ 1.22 billion towards the partnership’s vital work on access to COVID-19 treatments, tests, vaccines, and personal protective equipment.
Last month, President Ramaphosa of South Africa and Prime Minister Støre of Norway – in their roles as co-chairs of the ACT-Accelerator Facilitation Council – made a call to 55 countries to jointly support global efforts to end the COVID-19 crisis and contribute their ‘fair share’ to the ACT-Accelerator agencies’ US$ 16.8 billion urgent needs and end the COVID-19 crisis.
‘Fair shares’ are calculated based on the size of a country’s national economy and what they would gain from a faster recovery of the global economy and trade.
The pledge – announced by Finance Minister Christian Lindner at the G7 Finance Ministers’ Meeting today – takes Germany to its ‘fair share’ ask for this budget cycle and is accompanied by an additional US$ 253 million for supplementary measures for the in-country COVID-19 response, complementary to the ACT-A mission…Germany also exceeded its fair share for the 2020/21 budget cycle with US$ 2.5 billion provided across all of the ACT-Accelerator’s areas of work, a vital contribution to saving lives, protecting health systems and reducing the evolution of dangerous new variants…
COVID – Global Immunization Targets
Analysis: Is WHO’s aim to vaccinate 70% of world by June still realistic?
Reuters February 28, 2022
By Jennifer Rigby
LONDON, Feb 28 (Reuters) – Vaccinating 70% of the population in every country in the world against COVID-19 by mid-2022 has been the World Health Organization’s (WHO) rallying cry to end the pandemic.
But recently, public health experts say that while boosting immunity globally remains essential, the figure is neither achievable nor meaningful.
It has always been ambitious: Currently, just 12% of people in low-income nations have had one shot, according to Our World In Data. Earlier targets set by WHO – to reach 10% by September 2021, for example – were also missed.
WHO head of immunization Kate O’Brien said 70% remained more than just a “rallying cry”, even though some well-equipped countries with plenty of vaccines have also struggled to reach it. “We are calling for countries to be serious about their actions towards achieving that target, while acknowledging that – on a country-by-country basis – there may be a rationale why that target is not specifically suited to that country,” she told Reuters.
Gavi, the Vaccine Alliance – WHO’s partner in the COVAX initiative aimed at getting shots to the world’s poorest – has pulled back from the “one-size-fits-all” 70% focus. At a virtual briefing last week with WHO Africa, Aurelia Nguyen, managing director of COVAX within Gavi, said it was important to instead “meet the targets that countries have set for themselves, whether it’s in line with the 70% WHO target or a lower or a higher target.”
Reservations about the 70% target are a further sign that ending the pandemic globally may be a trickier, and longer, challenge than many had hoped. Documents from a high-level internal UN meeting held earlier this month, reviewed by Reuters, showed eight countries that were extremely unlikely to reach the target by June 2022, and had been identified for “immediate focus”: Afghanistan, the Democratic Republic of Congo, Ethiopia, Ghana, Kenya, Nigeria, Sierra Leone and Sudan. A further 26, including Yemen, Uganda and Haiti, are also in need of “concerted support”, the document said.
NEVER JUST A MAGIC NUMBER
However, there is a bigger issue the WHO is focusing on O’Brien said. “The question in the here and now, with Omicron ripping through the population around the world and continuing to do that … does 70% still hold?” she said. The figure was never a “magic number”, she said, but just an assessment of risk, something to aim for that could – optimistically – keep the virus under control.
But new evidence showing that the vaccines only have a limited impact on transmission, alongside the ability of the Omicron variant to infect previously vaccinated or infected people, suggests that achieving that level of population immunity and therefore stopping the spread of the virus is a fading hope.
“We are in the process of looking at scenarios of how the pandemic might play out”, O’Brien said. “Obviously across the scenarios, the role of the vaccines, the target of the 70%, the goal of transmission reduction, would have to be evaluated.” For example, setting higher targets among at-risk groups may be important to prevent hospitalisations and deaths, she added.
But some public health experts said the initial target was now largely symbolic. Edward Kelley, former director of health services at WHO and now global health officer at ApiJect, said the 70% had been based on what science said was needed to manage transmission, which had been blown out of the water by Omicron. “Of course we need to continue to raise immunity levels everywhere”, he said. “But the target is being kept at the moment because the international community does not have anything else to cling to.”…
::::::
Coronavirus [COVID-19] – WHO
Public Health Emergency of International Concern (PHEIC)
https://www.who.int/emergencies/diseases/novel-coronavirus-2019
Weekly Epidemiological and Operational updates
Last update: 25 Feb 2022
Confirmed cases :: 440 807 756
Confirmed deaths :: 5 978 096
Vaccine doses administered: 10 585 766 316
::::::
Weekly epidemiological update on COVID-19 – 1 March 2022
Overview
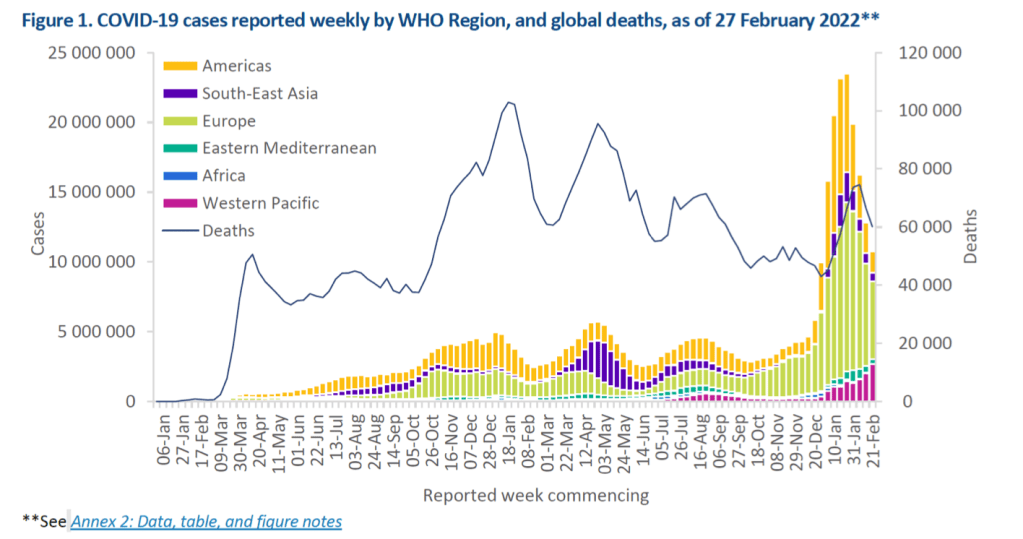
Globally, during the week of 21 through 27 February 2022, the number of new COVID-19 cases and deaths continued to decline by 16% and 10% respectively, as compared to the previous week. Across the six WHO regions, over 10 million new cases and over 60 000 new deaths were reported. As of 27 February 2022, over 433 million confirmed cases and over 5.9 million deaths have been reported globally.
At the regional level, the Western Pacific Region reported a 32% increase in the number of new weekly cases while all other regions reported decreases. The number of new weekly deaths increased in the Western Pacific (+22%) and the Eastern Mediterranean (+4%) Regions, whilst a decreasing trend have been reported by the Regions of Africa (-59%), South-East Asia (-18%), Europe (-13%) and Americas (-8%).
In this edition, we provide updates on the geographic distribution of circulating SARS-CoV-2 variants of concern (VOCs), including the spread and prevalence of the Omicron variant.
WHO Director General Speeches [selected]
https://www.who.int/director-general/speeches
Selected
3 March 2022
Speech
WHO Director-General’s remarks at Session 2 – Potential Opportunities for Innovation and Collaboration COVID-19 Dialogue with Ministers of Health – 3 March 2022
3 March 2022
Speech
WHO Director-General’s opening remarks at the Member State Information Session on COVID-19 – 3 March 2022
2 March 2022
Speech
WHO Director-General’s opening remarks at the media briefing on COVID-19 – 2 March 2022
Status of COVID-19 Vaccines within WHO EUL/PQ evaluation process 23 December 2021
[Full scale view available at title link above]
[Updated on 02 March 2022]
COVID Vaccines/Therapeutics – Developer/Manufacturer Announcements
[Selected press releases/announcements from organizations from WHO EUL/PQ listing above and other organizations]
AstraZeneca
Press Releases – No new digest announcements identified
Bharat Biotech
Press Releases – No new digest announcements identified
BioCubaFarma – Cuba
Últimas Noticias – Website not leading at inquiry
Biontech
Press Releases – No new digest announcements identified
CanSinoBIO
News – [Website not responding at inquiry]
Clover Biopharmaceuticals – China
News – No new digest announcements identified
Curevac [Bayer Ag – Germany]
News – Website not responding at inquiry
Gamaleya National Center
Latest News and Events – See Russia below and under Ukraine above
IMBCAMS, China
Home – Website not responding at inquiry
Janssen/JNJ
Press Releases – No new digest announcements identified
Moderna
Press Releases – No new digest announcements identified
Novavax
Press Releases
Novavax Announces Extended Durability of Protection Against Infection and Disease in United Kingdom COVID-19 Vaccine Phase 3 Clinical Trial
Feb 28, 2022
Pfizer
Recent Press Releases
03.02.2022
Pfizer Granted FDA Breakthrough Therapy Designation for Respiratory Syncytial Virus (RSV) Vaccine Candidate for the Prevention of RSV in Infants from Birth up to Six Months of Age by Active Immunization of Pregnant Women
Sanofi Pasteur
Press Releases – No new digest announcements identified
Serum Institute of India
NEWS & ANNOUNCEMENTS – No new digest announcements identified
Shifa Pharmed [Iran]
http://shafapharmed.com/
No news page identified.
Sinopharm/WIBPBIBP
News – No new digest announcements identified
Sinovac
Press Releases
Brazilian Study: Due to the vaccination roll-out applied to elderly: 167,000 hospitalizations and 77,000 lives were avoided
2022/03/04
Vector State Research Centre of Viralogy and Biotechnology
Home – No new digest announcements identified
Zhifei Longcom, China
[Anhui Zhifei Longcom Biologic Pharmacy Co., Ltd.]
[No website identified]
::::::
GSK
Press releases for media
GSK provides further update on phase III RSV maternal vaccine candidate programme
28 February 2022
Merck
News releases –
Dr. Julie L. Gerberding to Retire from Merck
March 1, 2022
KENILWORTH, N.J.–(BUSINESS WIRE)– Merck (NYSE: MRK), known as MSD outside the United States and Canada, today announced that Dr. Julie L. Gerberding, chief patient officer and executive vice president, population health and sustainability, will be retiring from Merck in May 2022. Earlier today, the Foundation for the National Institutes of Health (FNIH) announced that Dr. Gerberding will become CEO of the FNIH on May 16…
Novartis
News – No new digest announcements identified
SK Biosciences
Press releases – No new digest announcements identified
Valneva
Press Releases
March 1, 2022
Valneva Receives Emergency Use Authorization from Bahrain for its Inactivated COVID-19 Vaccine VLA2001
UNICEF COVID-19 Vaccine Market Dashboard :: Agreements Table Accessed 05 Mar 2022
An overview of information collected from publicly announced bilateral and multilateral supply agreements [no new agreements since 10/22/2021 reported]
COVID-19 Global Targets and Progress Tracker – IMF
The COVID-19 Global Targets and Progress Tracker presents a consolidated view of the progress towards global COVID-19 targets, barriers in access to COVID-19 tools, and delivery of donor pledges. The global targets presented in the Tracker are based on an alignment of the targets identified in the IMF Pandemic Proposal, ACT-A Strategic Plan & Budget, and the US-hosted Global C19 Summit, and as such have been reaffirmed by multilateral institutions and global leaders. We will continue to enhance the tracker as we improve our data collection efforts.
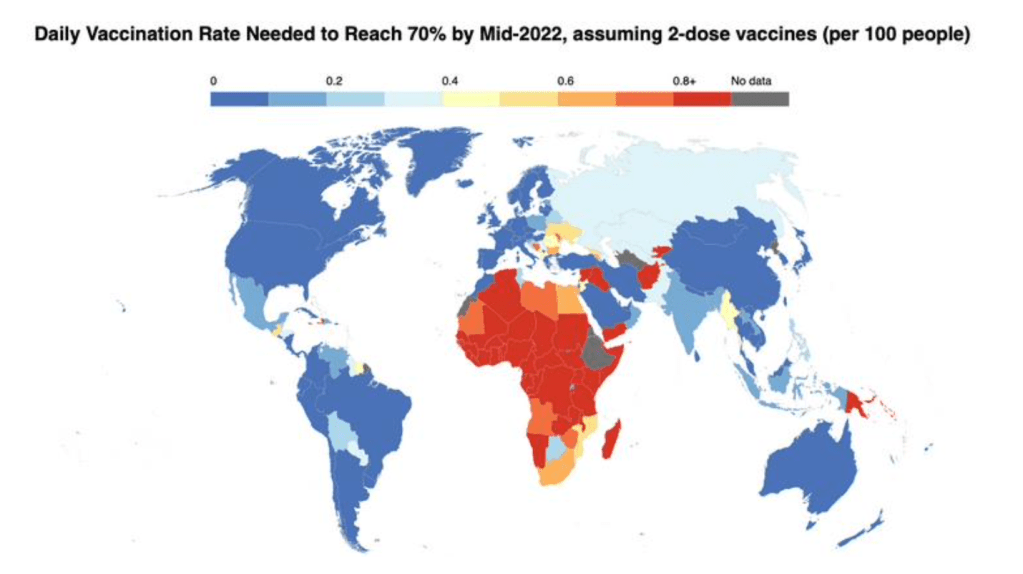
Global Dashboard on COVID-19 Vaccine Equity
The Dashboard is a joint initiative of UNDP, WHO and the University of Oxford with cooperation across the UN system, anchored in the SDG 3 Global Action Plan for Healthy Lives and Well-being for All.
Dashboard on Vaccine Equity [accessed 05 Mar 2022]: https://data.undp.org/vaccine-equity/
See also visualization on Vaccine Access and Vaccine Affordability
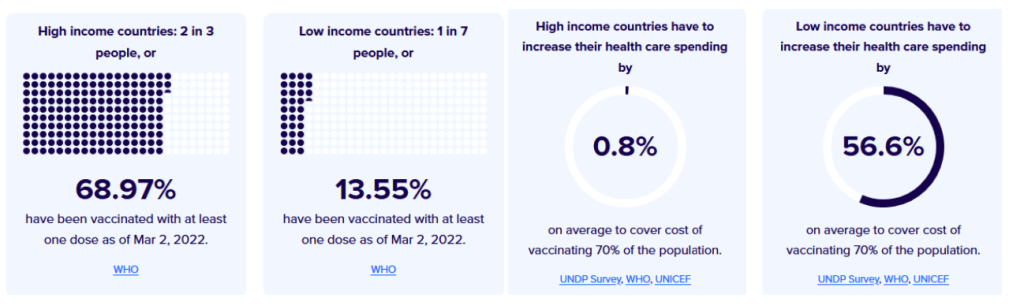
Duke – Launch and Scale Speedometer
The Race for Global COVID-19 Vaccine Equity
A flurry of nearly 200 COVID-19 vaccine candidates are moving forward through the development and clinical trials processes at unprecedented speed; more than ten candidates are already in Phase 3 large-scale trials and several have received emergency or limited authorization. Our team has aggregated and analyzed publicly available data to track the flow of procurement and manufacturing and better understand global equity challenges. We developed a data framework of relevant variables and conducted desk research of publicly available information to identify COVID vaccine candidates and status, deals and ongoing negotiations for procurement and manufacturing, COVID burden by country, and allocation and distribution plans. We have also conducted interviews with public officials in key countries to better understand the context and challenges facing vaccine allocation and distribution
[accessed 24 July 2021]
See our COVID Vaccine Purchases research
See our COVID Vaccine Manufacturing research
See our COVID Vaccine Donations & Exports research
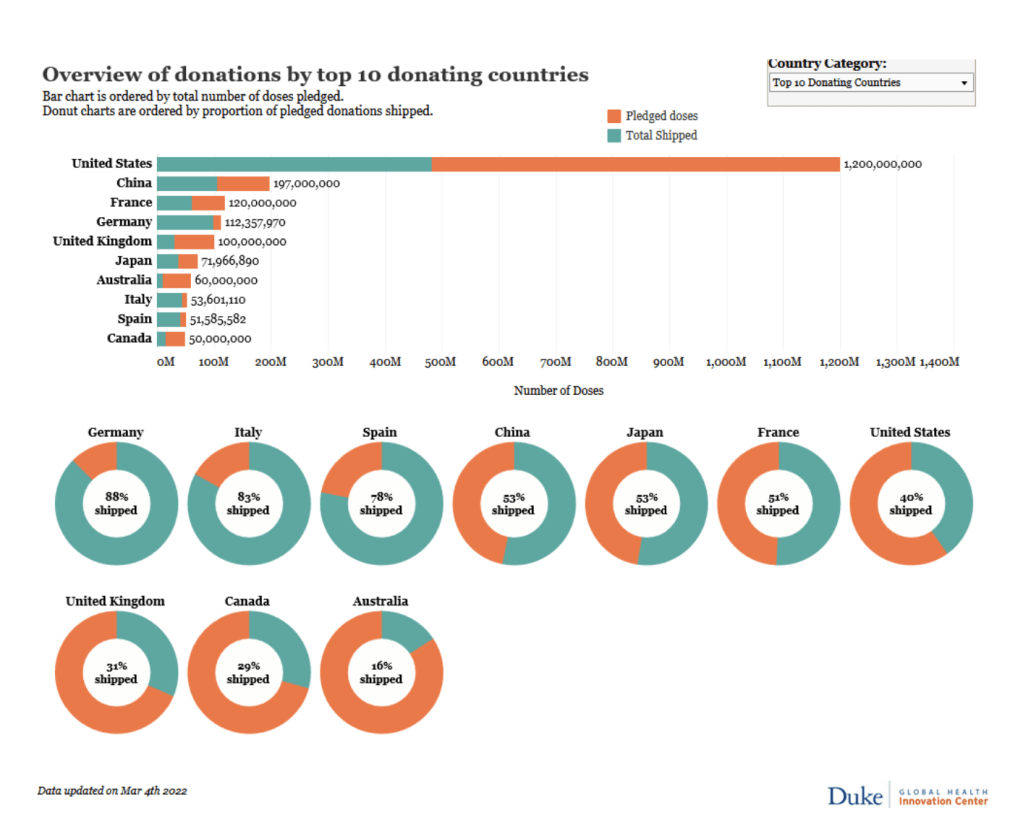
COVID Vaccines – OCHA:: HDX
COVID-19 Data Explorer: Global Humanitarian Operations
COVID-19 Vaccine Roll-out
05 Mar 2022 | COVAX (WHO,GAVI,CEPI), UNDESA, Press Reports | DATA
Global COVID-19 Figures: 4410M total confirmed cases; 6.0M total confirmed deaths
Global vaccines administered: 10.7B
Number of Countries: 28
COVAX Allocations Round 4-9 (Number of Doses): 170M
COVAX Delivered (Number of Doses): 260M
Other Delivered (Number of Doses): 250M
Total Delivered (Number of Doses): 520M
Total Administered (Number of Doses): 320M
Multilateral Leaders Task Force on COVID-19 [IMF, World Bank Group, WHO, WTO]
https://data.covid19taskforce.com/data
A global effort to help developing countries access and deliver COVID-19 vaccines, testing, and therapeutics, as they work to end the pandemic and boost economic recovery.
The International Monetary Fund, World Bank Group, World Health Organization and World Trade Organization have joined forces to accelerate access to COVID-19 vaccines, therapeutics and diagnostics by leveraging multilateral finance and trade solutions, particularly in low- and middle-income countries.
Website accessed 05 Mar 2022: https://data.covid19taskforce.com/data The global view below is complemented by country-specific dashboards here.
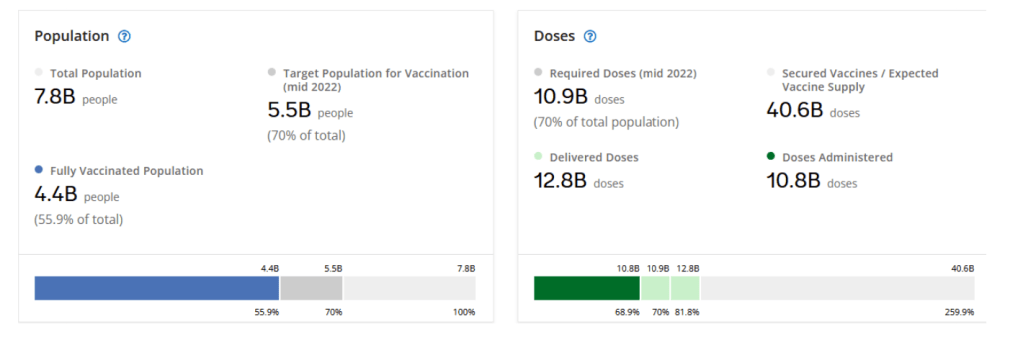
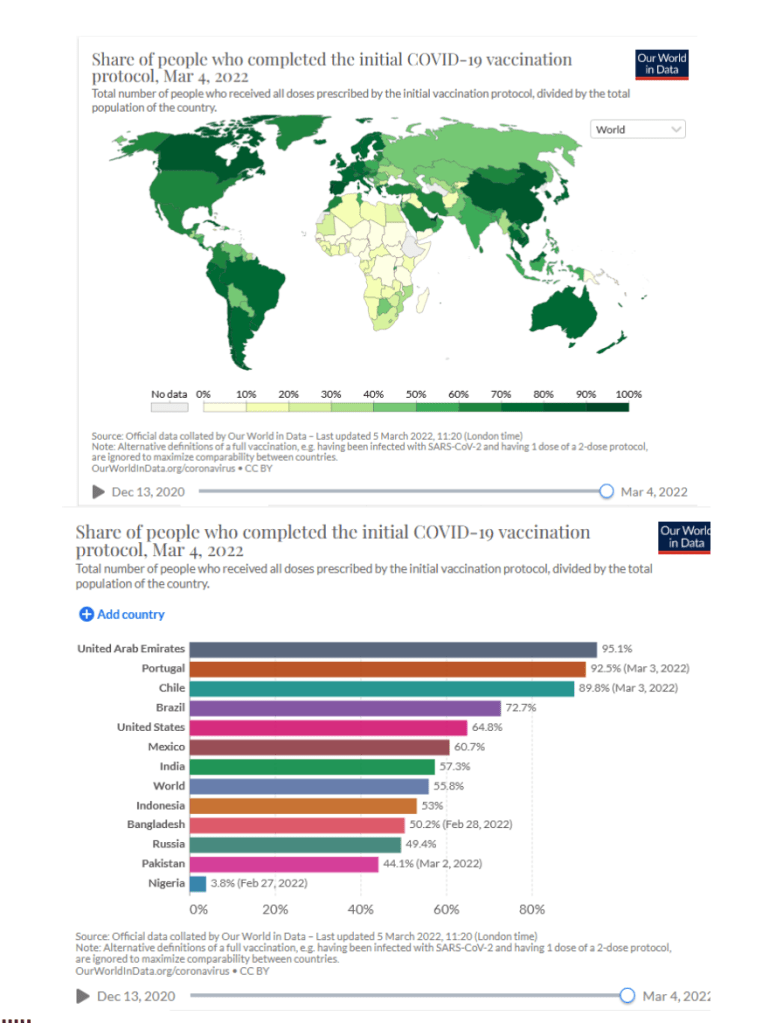
Our World in Data
Coronavirus (COVID-19) Vaccinations [Accessed 05 Mar 2022]
63.2% of the world population has received at least one dose of a COVID-19 vaccine.
10.84 billion doses have been administered globally, and 22.03 million are now administered each day.
Only 13% of people in low-income countries have received at least one dose.
U.S.: COVID-19 Vaccines – Announcements/Regulatory Actions/Deployment
HHS
News
Readout of Secretary Becerra’s Virtual Roundtable on Increasing Routine Vaccinations
February 24, 2022 | News Release
BARDA – U.S. Department of HHS [to 05 Mar 2022]
https://aspr.hhs.gov/newsroom/Pages/NewsRoomHome.aspx
News
No new digest content identified.
::::::
FDA
Press Announcements
No new digest content identified.
Vaccines and Related Biological Products Advisory Committee– FDA
https://www.fda.gov/advisory-committees/blood-vaccines-and-other-biologics/vaccines-and-related-biological-products-advisory-committee
Calendar
No calendar announcements identified.
::::::
White House [U.S.] [to 05 Mar 2022]
Briefing Room – Selected Major COVID Announcements
National COVID-19 Preparedness Plan
March 01, 2022
Today, the U.S. government is releasing the National COVID-19 Preparedness Plan – which will enable America to move forward safely, sustaining and building on the progress we’ve made over the past 13 months. This plan lays out the roadmap to help us fight COVID-19 in the future as we begin to get back to our more normal routines. We look to a future when Americans no longer fear lockdowns, shutdowns, and our kids not going to school. It’s a future when the country relies on the powerful layers of protection we have built and invests in the next generation of tools to stay ahead of this virus.
The President’s National COVID-19 Preparedness Plan focuses on four key goals:
First, protect against and treat COVID.
Second, prepare for any new variants.
Third, prevent economic and school shutdowns.
Fourth, vaccinate the world and save lives…
Memorandum on Maximizing Assistance to Respond to COVID-19
March 01, 2022 • Presidential Actions
afely, sustaining and building on the progress we’ve made over the past 13 months…
U.S. Department of State [to 05 Mar 2022]
https://www.state.gov/coronavirus/releases/
Media Notes
No new digest content identified.
USAID [to 05 Mar 2022]
https://www.usaid.gov/news-information/press-releases/2021
Selected Press Releases, Statements, Announcements
News
Administrator Samantha Power at the German Marshall Fund’s Conversation On “Strengthening Crisis Support in Ukraine”
March 3, 2022
So I did travel as quickly as I could to the Ukrainian-Polish border. And we’ve been planning for — around a number of scenarios for many months, prepositioning stockpiles, getting the organization the World Food Programme, which had been active in Ukraine back when the conflict broke out in Crimea in Eastern Ukraine, but then had gotten out about three years ago. Getting them to reestablish a presence there, knowing that a conflict unfortunately was likely, that a Russian invasion was likely. And so part of what I was up to was checking in with our DART team, with our team of forty people who are these kind of crack emergency responders that help coordinate among the various agencies. The flow of goods and people across border crossings along the front-line states…
Europe: COVID-19 Vaccines – Announcements/Regulatory Actions/Deployment
European Medicines Agency
News & Press Releases
News: Regulation on EMA’s extended mandate becomes applicable (new)
Last updated: 01/03/2022
The regulation reinforcing EMA’s role in crisis preparedness and management of medicinal products and medical devices becomes applicable as of today, 1 March 2022. It puts some of the structures and processes established by EMA during the COVID-19 pandemic on a more permanent footing, while entrusting several new tasks to the Agency.
EMA is now responsible for monitoring medicine shortages that might lead to a crisis situation, as well as reporting shortages of critical medicines during a crisis. The Agency will also coordinate responses of EU / EEA countries to shortages of critical medical devices and in-vitro diagnostics in crisis situations, after an initial transition period up to 2 February 2023.
Over the next few weeks and months, EMA will set up a number of new bodies and formalise existing ones to manage the new tasks…
::::::
European Centre for Disease Prevention and Control
https://www.ecdc.europa.eu/en
Latest Updates [Selected]
No new digest content identified.
::::::
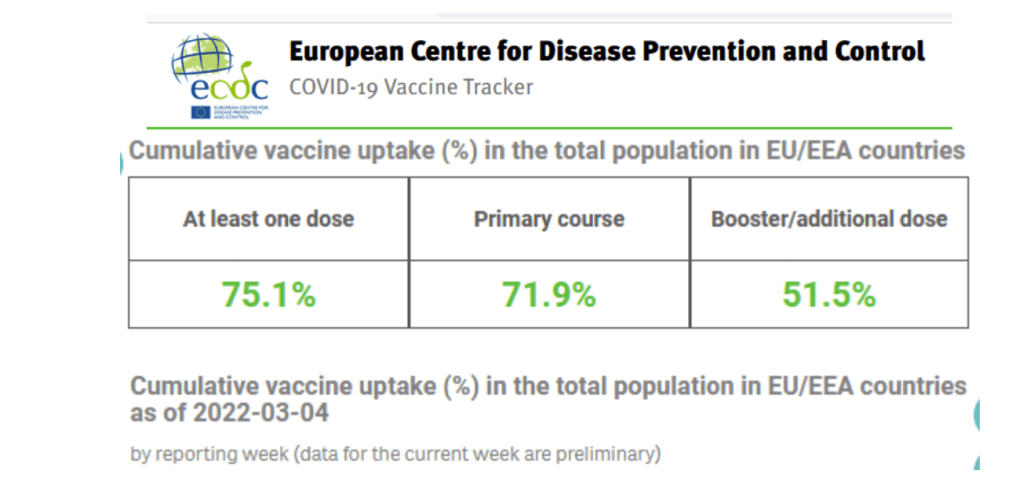
Accessed 05 Mar 2022
https://vaccinetracker.ecdc.europa.eu/public/extensions/COVID-19/vaccine-tracker.html#uptake-tab
::::::
European Commission
https://ec.europa.eu/commission/presscorner/home/en
Press release 4 March 2022
Commission suspends cooperation with Russia on research and innovation
[See Ukraine above for detail]
Press release 2 March 2022
Ukraine: Commission proposes temporary protection for people fleeing war in Ukraine and guidelines for border checks
Today, the Commission is proposing to activate the Temporary Protection Directive to offer quick and effective assistance to people fleeing the war in Ukraine.
Press release 2 March 2022
EU budget: Commission publishes guidance on the conditionality mechanism
Today the European Commission adopted its guidelines on the general regime of conditionality, which aims to protect the EU budget against breaches of the principles of the rule of law.
Speech 1 March 2022
Speech by President von der Leyen at the European Parliament Plenary on the Russian aggression against Ukraine
This is a moment of truth for Europe…
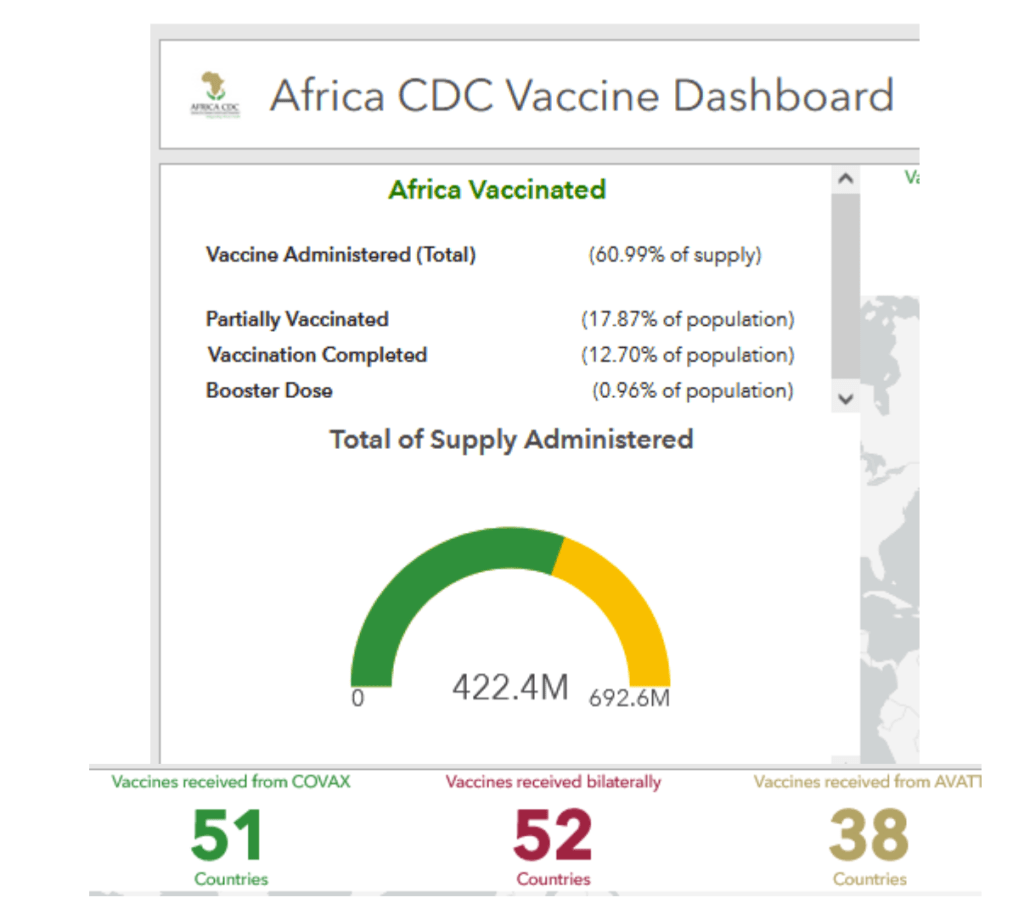
Africa: COVID-19 – Announcements/Regulatory Actions/Deployment
Accessed 05 Mar 2022. Full scale, interactive dashboard available at:
https://africacdc.org/covid-19-vaccination/