New England Journal of Medicine
October 7, 2021 Vol. 385 No. 15
http://www.nejm.org/toc/nejm/medical-journal
Protection of BNT162b2 Vaccine Booster against Covid-19 in Israel Y.M. Bar-On and Others
New England Journal of Medicine
October 7, 2021 Vol. 385 No. 15
http://www.nejm.org/toc/nejm/medical-journal
Protection of BNT162b2 Vaccine Booster against Covid-19 in Israel Y.M. Bar-On and Others
New England Journal of Medicine
October 7, 2021 Vol. 385 No. 15
http://www.nejm.org/toc/nejm/medical-journal
New England Journal of Medicine
October 7, 2021 Vol. 385 No. 15
http://www.nejm.org/toc/nejm/medical-journal
Audio Interview: Are Covid-19 Vaccine Boosters Necessary? E.J. Rubin, L.R. Baden, and S. Morrissey
Pediatrics
Vol. 148, Issue 4 1 Oct 2021
https://pediatrics.aappublications.org/
Articles
Parents’ Intentions and Perceptions About COVID-19 Vaccination for Their Children: Results From a National Survey
Peter G. Szilagyi, Megha D. Shah, Jeanne R. Delgado, Kyla Thomas, Nathalie Vizueta, Yan Cui, Sitaram Vangala, Rashmi Shetgiri, Arie Kapteyn
Pediatrics, Oct 2021, 148 (4) e2021052335
Pediatrics
Vol. 148, Issue 4 1 Oct 2021
https://pediatrics.aappublications.org/
Community SARS-CoV-2 Surge and Within-School Transmission
Kanecia O. Zimmerman, M. Alan Brookhart, Ibukunoluwa C. Kalu, Angelique E. Boutzoukas, Kathleen A. McGann, Michael J. Smith, Gabriela M. Maradiaga Panayotti, Sarah C. Armstrong, David J. Weber, Ganga S. Moorthy, Daniel K. Benjamin Jr for The ABC Science Collaborative
Pediatrics, Oct 2021, 148 (4) e2021052686
Pediatrics
Vol. 148, Issue 4 1 Oct 2021
https://pediatrics.aappublications.org/
Pediatrics Perspectives
Open Access
Important Considerations for COVID-19 Vaccination of Children With Developmental Disabilities
Sarah C. Tinker, Mary E Cogswell, Georgina Peacock, A. Blythe Ryerson
Pediatrics, Oct 2021, 148 (4) e2021053190
PLoS Medicine
http://www.plosmedicine.org/
(Accessed 09 Oct 2021)
The global burden of Plasmodium vivax malaria is obscure and insidious
Katherine E. Battle, J. Kevin Baird
Collection Review | published 07 Oct 2021 PLOS Medicine
https://doi.org/10.1371/journal.pmed.1003799
PLoS Medicine
http://www.plosmedicine.org/
(Accessed 09 Oct 2021)
COVID-19 vaccination in Sindh Province, Pakistan: A modelling study of health impact and cost-effectiveness
Carl A. B. Pearson, Fiammetta Bozzani, Simon R. Procter, Nicholas G. Davies, Maryam Huda, Henning Tarp Jensen, Marcus Keogh-Brown, Muhammad Khalid, Sedona Sweeney, Sergio Torres-Rueda, CHiL COVID-19 Working Group , CMMID COVID-19 Working Group , Rosalind M. Eggo, Anna Vassall, Mark Jit
Research Article | published 04 Oct 2021 PLOS Medicine
https://doi.org/10.1371/journal.pmed.1003815
PLoS Neglected Tropical Diseases
http://www.plosntds.org/
Viewpoints
Dengue outbreaks in the COVID-19 era: Alarm raised for Asia
Xinting Lu, Hilary Bambrick, Puntani Pongsumpun, Pandji Wibawa Dhewantara, Do Thi Thanh Toan, Wenbiao Hu
| published 08 Oct 2021 PLOS Neglected Tropical Diseases
https://doi.org/10.1371/journal.pntd.0009778
PLoS One
http://www.plosone.org/
[Accessed 09 Oct 2021]
Research Article
Considering equity in global health collaborations: A qualitative study on experiences of equity
Marlyn C. Faure, Nchangwi S. Munung, Ntobeko A. B. Ntusi, Bridget Pratt, Jantina de Vries
| published 07 Oct 2021 PLOS ONE
https://doi.org/10.1371/journal.pone.0258286
PNAS – Proceedings of the National Academy of Sciences of the United States
September 28, 2021; vol. 118 no. 39
https://www.pnas.org/content/118/39
Commentaries
Origin, transmission, and evolution of plague over 400 y in Europe
Rémi Barbieri
PNAS September 28, 2021 118 (39) e2114241118; https://doi.org/10.1073/pnas.2114241118
Engineering
PNAS – Proceedings of the National Academy of Sciences of the United States
September 28, 2021; vol. 118 no. 39
https://www.pnas.org/content/118/39
Transdermal vaccination via 3D-printed microneedles induces potent humoral and cellular immunity
Cassie Caudill, Jillian L. Perry, Kimon Iliadis, Addis T. Tessema, Brian J. Lee, Beverly S. Mecham, Shaomin Tian, and Joseph M. DeSimone
PNAS September 28, 2021 118 (39) e2102595118; https://doi.org/10.1073/pnas.2102595118
PNAS – Proceedings of the National Academy of Sciences of the United States
September 28, 2021; vol. 118 no. 39
https://www.pnas.org/content/118/39
Pharmacology
Open Access
Deep learning identifies synergistic drug combinations for treating COVID-19
Wengong Jin, Jonathan M. Stokes, Richard T. Eastman, Zina Itkin, Alexey V. Zakharov, James J. Collins, Tommi S. Jaakkola, and Regina Barzilay
PNAS September 28, 2021 118 (39) e2105070118; https://doi.org/10.1073/pnas.2105070118
Science Translational Medicine
Volume 13| Issue 614| 6 Oct 2021
https://www.science.org/toc/stm/current
Focus
No one is safe until we are all safe
BY Sarah Gilbert, Richard Hatchett
06 Oct 2021
Open Access
Ending the pandemic will require the world to shift its focus to equitably deploying and optimizing the use of vaccines now being produced at scale. In the second half of 2021, with a number of COVID-19 vaccines being produced, the focus must shift to equitable vaccine deployment and optimum use based on safety and effectiveness data.
Social Science & Medicine
Volume 285 September 2021
https://www.sciencedirect.com/journal/social-science-and-medicine/vol/285/suppl/C
Research article Abstract only
“Not a free version of a broken system:” Medical humanitarianism and immigrant health justice in the United States
Erin Hoekstra
Article 114287
Vaccine
Volume 39, Issue 40 Pages 5727-6014 (24 September 2021)
https://www.sciencedirect.com/journal/vaccine/vol/39/issue/40
Discussion Full text access
Making COVID-19 vaccinations accessible for people with disabilities
Sara Rotenberg, Matthew B. Downer, Jane Cooper
Pages 5727-5728
Vaccine
Volume 39, Issue 40 Pages 5727-6014 (24 September 2021)
https://www.sciencedirect.com/journal/vaccine/vol/39/issue/40
Research article Open access
The role of maturity in adolescent decision-making around HPV vaccination in France
E. Karafillakis, P. Peretti-Watel, P. Verger, T. Chantler, H.J. Larson
Pages 5741-5747
Abstract
Mothers are often responsible for vaccination decisions in the household. However, their confidence in certain vaccines such as Human Papillomavirus (HPV) vaccines is eroding in some countries. France is one of the countries with the lowest HPV vaccine uptake in Europe, with parents delaying or refusing the vaccine for their adolescent daughters due to safety- and effectiveness-related concerns. Although parental consent is required for vaccination, adolescents’ involvement in HPV vaccination decision-making could improve vaccine uptake, with self-consent procedures already introduced in some countries. Adolescents’ capacity to engage in decision-making is influenced by their maturity and autonomy in health. This study explored the role of maturity in decision-making around HPV vaccination in France through qualitative interviews with adolescent girls (n = 24) and their mothers (n = 21) and two focus groups with adolescent girls (n = 12). A codebook approach to thematic analysis revealed that adolescent girls’ involvement in HPV decision-making is a process that evolved with maturity. As adolescents progressed towards maturity at different speeds, some expressed childlike traits such as impulsive decisions and others described more rational, reflective decision-making. Despite these differences, most adolescents in this study described a passive role in HPV vaccination decision-making, following their parents’ lead. However, their expressed desire for information and involvement in discussions indicates that their lack of engagement may not only be due to a lack of maturity but also a result of mothers and doctors excluding them from getting involved. Furthermore, as health behaviours are shaped during adolescence, the influence of vaccine hesitant mothers on their daughters’ own views and beliefs could be significant, together with exposure to regular controversies in the mainstream media. Individualised approaches to engage adolescents in decision-making around their own health are needed, for example through strengthening discussions and information around HPV vaccination with parents and doctors.
Vaccine
Volume 39, Issue 40 Pages 5727-6014 (24 September 2021)
https://www.sciencedirect.com/journal/vaccine/vol/39/issue/40
Research article Open access
COVID-19 vaccine uptake among healthcare workers in the fourth country to authorize BNT162b2 during the first month of rollout
Mazin Barry, Mohamad-Hani Temsah, Fadi Aljamaan, Basema Saddik, … Jaffar A. Al-Tawfiq
Pages 5762-5768
Vaccine
Volume 39, Issue 40 Pages 5727-6014 (24 September 2021)
https://www.sciencedirect.com/journal/vaccine/vol/39/issue/40
Research article Full text access
COVID-19 vaccination in the Federal Bureau of Prisons, December 2020—April 2021
Liesl M. Hagan, Charles Dusseau, Michael Crockett, Tami Rodriguez, Michael J. Long
Pages 5883-5890
Vaccine
Volume 39, Issue 40 Pages 5727-6014 (24 September 2021)
https://www.sciencedirect.com/journal/vaccine/vol/39/issue/40
Research article Full text access
Safety of components and platforms of COVID-19 vaccines considered for use in pregnancy: A rapid review
Agustín Ciapponi, Ariel Bardach, Agustina Mazzoni, Tomás Alconada, … Pierre M. Buekens
Pages 5891-5908
Vaccine
Volume 39, Issue 40 Pages 5727-6014 (24 September 2021)
https://www.sciencedirect.com/journal/vaccine/vol/39/issue/40
Research article Abstract only
Which young women are not being vaccinated against HPV? Cross-sectional analysis of a UK national cohort study
Helen Bedford, Nicola Firman, Jo Waller, Laura Marlow, … Carol Dezateux
Pages 5934-5939
Vaccine
Volume 39, Issue 40 Pages 5727-6014 (24 September 2021)
https://www.sciencedirect.com/journal/vaccine/vol/39/issue/40
Research article Abstract only
The public’s role in COVID-19 vaccination: Human-centered recommendations to enhance pandemic vaccine awareness, access, and acceptance in the United States
Monica Schoch-Spana, Emily K. Brunson, Rex Long, Alexandra Ruth, … Alexandre White
Pages 6004-6012
Vaccines
https://www.mdpi.com/journal/vaccines
Open Access Article
COVID-19 Vaccination Attitudes, Perceptions, and Side Effect Experiences in Malaysia: Do Age, Gender, and Vaccine Type Matter?
by Mohamed Hassan Elnaem et al.
Vaccines 2021, 9(10), 1156; https://doi.org/10.3390/vaccines9101156 (registering DOI) – 09 Oct 2021
Abstract
This study aimed to investigate the attitudes, perceptions, and experiences of side effects with the COVID-19 vaccines in Malaysia among participants in the National Vaccination Program. A cross-sectional survey was conducted among a sample of vaccine-eligible and vaccinated individuals in Malaysia between May […]
Vaccines
https://www.mdpi.com/journal/vaccines
Open Access Article
Predictors of COVID-19 Vaccination Campaign Success: Lessons Learnt from the Pandemic So Far. A Case Study from Poland
by Marcin Piotr Walkowiak and Dariusz Walkowiak
Vaccines 2021, 9(10), 1153; https://doi.org/10.3390/vaccines9101153 (registering DOI) – 09 Oct 2021
Abstract
The high effectiveness of a vaccination-promotion campaign, which may be measured by the number of those successfully convinced to get vaccinated, is a key factor in combating the COVID-19 pandemic. This, however, appears to be linked to the precise identification of the underlying causes for vaccine hesitancy behaviours. Based on a regression model (adjusted R2 of 0.78) analysing 378 sub-regions of Poland, we showed that such behaviours, even when going against the party agenda, can be indirectly yet precisely gauged predominantly through voting patterns. Additionally, education and population density were found to be positively related to low vaccine hesitancy, while markers of social exclusion, both external (employment rate) and psychological (voter turnout) ones, affected it negatively. In the second, follow-up part of our study, which analyses the changes that took place in two months (adjusted R2 of 0.53), we found a further increase in vaccination rate to be positively related to the number of those already vaccinated and to the political views of the population, and negatively related to its level of education. In both cases, there was a surprisingly weak relationship between the potential markers of accessibility and vaccination rate. In spite of the known overall differences in vaccination rates for different age and sex groups, these variables did not have any additional informative value in explaining the observed regional differences.
Vaccines
https://www.mdpi.com/journal/vaccines
Open Access Article
COVID-19 Vaccine Concerns about Safety, Effectiveness, and Policies in the United States, Canada, Sweden, and Italy among Unvaccinated Individuals
by Rachael Piltch-Loeb et al.
Vaccines 2021, 9(10), 1138; https://doi.org/10.3390/vaccines9101138 (registering DOI) – 06 Oct 2021
Abstract
Despite the effectiveness of the COVID-19 vaccine, global vaccination distribution efforts have thus far had varying levels of success. Vaccine hesitancy remains a threat to vaccine uptake. This study has four objectives: (1) describe and compare vaccine hesitancy proportions by country; (2) categorize […]
Vaccines
https://www.mdpi.com/journal/vaccines
Open Access Article
The Role of Attitudes, Norms, and Efficacy on Shifting COVID-19 Vaccine Intentions: A Longitudinal Study of COVID-19 Vaccination Intentions in New Zealand
by Jagadish Thaker and Somrita Ganchoudhuri
Vaccines 2021, 9(10), 1132; https://doi.org/10.3390/vaccines9101132 (registering DOI) – 04 Oct 2021
Abstract
While public intentions to get a COVID-19 vaccine have been shifting around the world, few studies track factors that help us understand and improve COVID-19 vaccine uptake. This study focuses on identifying changing public intentions to get a COVID-19 vaccine in New Zealand, […]
Value in Health
October 2021 Volume 24 Issue 10 p1391-1542
https://www.valueinhealthjournal.com/current
SYSTEMATIC LITERATURE REVIEW
How Much Is a Human Life Worth? A Systematic Review
Elena Keller, Jade E. Newman, Andreas Ortmann, Louisa R. Jorm, Georgina M. Chambers
Published online: May 25, 2021
p1531-1541
Highlights
What is already known about this topic?
Monetary valuation of a human life – the value of a statistical life (VSL) – is used in a broad range of policy areas resulting in a wide range of VSL estimates. Literature reviews and meta-analyses have generally focused on specific elicitation methods or sectors such as traffic or occupational risk. A review of VSL estimates using different elicitation methods across sectors would improve our understanding of recent developments and highlight future directions for research.
What does this article add to existing knowledge?
We included 120 studies which makes our study the most comprehensive review of VSL methods and estimates across sectors to date. The median of midpoint VSL estimates was $6.8 million, $8.7 million, and $5.3 million for the health, labor market, and transportation safety sectors, respectively. The variation in VSL depends mainly on the elicitation context (sector, developed/developing country, socio-economic status, etc) rather than the method used. Stated-preference techniques were the most common elicitation method.
What insights does this article provide for informing healthcare-related decision making?
We provide evidence that for policy evaluations context-specific VSL estimates, rather than one overarching average VSL estimate, ought to be used. Although stated- and revealed-preference elicitation methods yield comparable estimates within a sector, the VSL estimates for labor markets and developed countries tend to be higher. Overall, the VSL literature suggests that individuals value a life-year more highly than willingness-to-pay thresholds used in health technology assessment.
medRxiv
medRxiv is a free online archive and distribution server for complete but unpublished manuscripts (preprints) in the medical, clinical, and related health sciences. Preprints are preliminary reports of work that have not been certified by peer review. They should not be relied on to guide clinical practice or health-related behavior and should not be reported in news media as established information. medRxiv is for the distribution of preprints – complete but unpublished manuscripts – that describe human health research conducted, analyzed, and interpreted according to scientific principles…
https://www.medrxiv.org/content/about-medrxiv
[Accessed 09 Oct 2021]
Selected Content
COVID-19 Vaccine Effectiveness by Product and Timing in New York State
Eli Rosenberg, Vajeera Dorabwila, Delia Easton, Ursula Bauer, Jessica Kumar, Rebecca Hoen, Dina Hoefer, Meng Wu, Emily Lutterloh, Mary Beth Conroy, Danielle Greene, Howard A Zucker
medRxiv 2021.10.08.21264595; doi: https://doi.org/10.1101/2021.10.08.21264595
Estimating the effectiveness of first dose of COVID-19 vaccine against mortality in England: a quasi-experimental study
Charlotte Bermingham, Jasper Morgan, Daniel Ayoubkhani, Myer Glickman, Nazrul Islam, Aziz Sheikh, Jonathan Sterne, A. Sarah Walker, Vahé Nafilyan
medRxiv 2021.07.12.21260385; doi: https://doi.org/10.1101/2021.07.12.21260385
Estimating the effectiveness of first dose of COVID-19 vaccine against mortality in England: a quasi-experimental study
Charlotte Bermingham, Jasper Morgan, Daniel Ayoubkhani, Myer Glickman, Nazrul Islam, Aziz Sheikh, Jonathan Sterne, A. Sarah Walker, Vahé Nafilyan
medRxiv 2021.07.12.21260385; doi: https://doi.org/10.1101/2021.07.12.21260385
Delta variants of SARS-CoV-2 cause significantly increased vaccine breakthrough COVID-19 cases in Houston, Texas
Paul A. Christensen, Randall J. Olsen, S. Wesley Long, Sishir Subedi, James J. Davis, Parsa Hodjat, Debbie R. Walley, Jacob C. Kinskey, Matthew Ojeda Saavedra, Layne Pruitt, Kristina Reppond, Madison N. Shyer, Jessica Cambric, Ryan Gadd, Rashi M. Thakur, Akanksha Batajoo, Regan Mangham, Sindy Pena, Trina Trinh, Prasanti Yerramilli, Marcus Nguyen, Robert Olson, Richard Snehal, Jimmy Gollihar, James M. Musser
medRxiv 2021.07.19.21260808; doi: https://doi.org/10.1101/2021.07.19.21260808
Postvaccination SARS-CoV-2 infection among healthcare workers – A Systematic Review and meta-analysis
Saurabh Chandan, Shahab R. Khan, Smit Deliwala, Babu P. Mohan, Daryl Ramai, Ojasvini C. Chandan, Antonio Facciorusso
medRxiv 2021.10.04.21264542; doi: https://doi.org/10.1101/2021.10.04.21264542
Covid-19 vaccine perceptions in Senegal and in Mali: a mixed approach
Eleonore Fournier-Tombs, Massamba Diouf, Abdine Maiga, Sylvain Faye, Tidiane Ndoye, Lalla Haidara, Moussa Batchily, Céline Castet-Renard
medRxiv 2021.10.06.21264664; doi: https://doi.org/10.1101/2021.10.06.21264664
Adverse events of special interest for COVID-19 vaccines – background incidences vary by sex, age and time period and are affected by the pandemic
Fredrik Nyberg, Magnus Lindh, Lowie E.G.W. Vanfleteren, Niklas Hammar, Björn Wettermark, Johan Sundström, Ailiana Santosa, Brian K. Kirui, Magnus Gisslén
medRxiv 2021.10.04.21263507; doi: https://doi.org/10.1101/2021.10.04.21263507
Non-pharmaceutical interventions and vaccinating school children required to contain SARS-CoV-2 Delta variant outbreaks in Australia: a modelling analysis
George J Milne, Julian Carrivick, David Whyatt
medRxiv 2021.10.03.21264492; doi: https://doi.org/10.1101/2021.10.03.21264492
Using conditional inference to quantify interaction effects of socio-demographic covariates of US COVID-19 vaccine hesitancy
Ke Shen, Mayank Kejriwal
medRxiv 2021.10.02.21264456; doi: https://doi.org/10.1101/2021.10.02.21264456
COVID-19 data reporting systems in Africa reveal insights for pandemic preparedness
Seth D. Judson, Judith Torimiro, David M. Pigott, Apollo Maima, Ahmed Mostafa, Ahmed Samy, Peter Rabinowitz, Kevin Njabo
medRxiv 2021.10.01.21264385; doi: https://doi.org/10.1101/2021.10.01.21264385
The Brazilian Rare Genomes Project: validation of whole genome sequencing for rare diseases diagnosis
Antonio Victor Campos Coelho, Bruna Mascaro Cordeiro de Azevedo, Danielle Ribeiro Lucon, Maria Soares Nóbrega, Rodrigo de Souza Reis, Rodrigo Bertollo de Alexandre, Livia Maria Silva Moura, Gustavo Santos de Oliveira, Rafael Lucas Muniz Guedes, Marcel Pinheiro Caraciolo, Nuria Bengala Zurro, Murilo Castro Cervato, João Bosco de Oliveira Filho
medRxiv 2021.10.01.21264436; doi: https://doi.org/10.1101/2021.10.01.21264436
Think Tanks et al
Brookings [to 09 Oct 2021]
http://www.brookings.edu/
Oct 15
Upcoming Event
A proposal for long-term COVID-19 control
2:00 PM – 3:00 PM EDT
Online Only
Center for Global Development [to 09 Oct 2021]
http://www.cgdev.org/page/press-center
Accessed 09 Oct 2021
The Future of Globalization
October 8, 2021
Amidst the debate, fears, political polarization, and regrets surrounding globalization, we cannot ignore a central reality: much of it is not reversible or even resistable. As in other periods of human history where new connections are forged between geographies and civilizations—whether driven by empire building, technological change, regime change, or climate change-driven migration—Pandora’s Box, once opened, cannot be closed. We explore the major forces that will shape globalization in the future, and the policy and institutional changes needed globally and across a broad swath of countries.
Masood Ahmed and Nancy Lee
How Far Have Southeast Asian Countries Come with Digitizing Vaccination Certificates?
October 7, 2021
In May, we examined the possibility of Southeast Asian countries working together to create a regional COVID-19 Vaccination Certification (CVC) system. How far have Southeast Asian countries come in their CVC efforts? What form can certificates take and how can their authenticity be verified, given the limitations in infrastructure and capacity? Will mutual recognition of CVCs be possible when the type of vaccines and their doses differ significantly across countries in the region? We explore these questions, summarizing the discussions of a recent webinar on this very topic.
Azusa Sato and Anit Mukherjee
Are We Entering a New Age of Pandemics?
Publication
October 7, 2021
The first age of pandemics followed in the wake of farming, cities and trade, because infections leverage proximity and numbers to survive and evolve. After millennia of mass mortality, followed by two centuries of progress against plagues driven by sanitary and medical revolutions, will we allow a second age of pandemic death to flourish in the dense and connected world that progress has created? A poxed century can be avoided if we cooperate to respond.
Chatham House [to 09 Oct 2021]
https://www.chathamhouse.org/
Accessed 09 Oct 2021
Expert comment 5 October 2021
Policy failure on vaccines does not bode well for COP26
Global leadership is needed to tackle the climate crisis, but a failure in solidarity on COVID-19 leaves the creditability of world leaders in huge doubt.
Robert Yates
Director, Global Health Programme; Executive Director, Centre for Universal Health
CSIS
https://www.csis.org/
Accessed 09 Oct 2021
[No new digest content identified]
Kaiser Family Foundation
https://www.kff.org/search/?post_type=press-release
October 8, 2021 News Release
As the COVID-19 Pandemic Evolves, Disparities in Cases and Deaths for Black and Hispanic People Have Narrowed
As the COVID-19 pandemic’s focus shifts from urban to rural areas, and more people resume public activities, a new KFF analysis of case and death data from the Centers for Disease Control and Prevention reveals narrower disparities affecting Black and Hispanic people compared to White people now than earlier in…
October 1, 2021 News Release
The Decline in COVID-19 Deaths Among Nursing Home Residents and Staff Reversed Course Amid the Surging Delta Variant This Summer
The months-long decline in COVID-19 deaths among nursing home residents and staff reversed course this summer as the Delta variant dominated, with mortality increasing five-fold from 350 deaths in July to nearly 1,800 in August, finds a new KFF analysis. The analysis also finds increases in nursing home COVID-19 cases…
September 30, 2021 News Release
Nearly Half of Parents of Adolescents Ages 12-17 Say Their Child Got a COVID-19 Vaccine Already; a Third of Parents of Children Ages 5-11 Say Their Child Will Get Vaccinated “Right Away” Once Eligible
Nearly half (48%) of parents of vaccine-eligible children ages 12-17 now say their child has received at least one dose of a COVID-19 vaccine, a new KFF Vaccine Monitor report shows. Another 15% of those parents now say they want to “wait and see” how the vaccine works for others…
September 28, 2021 News Release
Surging Delta Variant Cases, Hospitalizations, and Deaths Are Biggest Drivers Of Recent Uptick in U.S. COVID-19 Vaccination Rates
Large Majorities of Americans, Both Vaccinated and Not, Say COVID-19 is Likely to Persist at Lower Levels and Be Something the U.S. Will “Learn to Live With” like Seasonal Flu More than 7 in 10 adults (72%) in the U.S. now report that they are at least partially vaccinated against…
September 27, 2021 News Release
As PEPFAR Nears its Two-Decade Mark, New Analysis Finds That Mortality Declined Substantially in PEPFAR Countries Over the Course of the Program
A new KFF analysis finds the President’s Emergency Plan for AIDS Relief (PEPFAR) program was associated with large declines in mortality in PEPFAR recipient countries since its creation in 2003. The new analysis takes a closer look at PEPFAR’s health impact by assessing the all-cause mortality rate in 90 PEPFAR…
Vaccines and Global Health: The Week in Review is a weekly digest summarizing news, events, announcements, peer-reviewed articles and research in the global vaccine ethics and policy space. Content is aggregated from key governmental, NGO, international organization and industry sources, key peer-reviewed journals, and other media channels. This summary proceeds from the broad base of themes and issues monitored by the Center for Vaccine Ethics & Policy in its work: it is not intended to be exhaustive in its coverage. You are viewing the blog version of our weekly digest, typically comprised of between 30 and 40 posts below all dated with the current issue date
.– Request an Email Summary: Vaccines and Global Health : The Week in Review is published as a single email summary, scheduled for release each Saturday evening before midnight (EDT in the U.S.). If you would like to receive the email version, please send your request to david.r.curry@centerforvaccineethicsandpolicy.org.
– pdf version: A pdf of the current issue is available here:
– blog edition: comprised of the approx. 35+ entries posted below.
– Twitter: Readers can also follow developments on twitter: @vaxethicspolicy.
.
– Links: We endeavor to test each link as we incorporate it into any post, but recognize that some links may become “stale” as publications and websites reorganize content over time. We apologize in advance for any links that may not be operative. We believe the contextual information in a given post should allow retrieval, but please contact us as above for assistance if necessary.
Support this knowledge-sharing service: Your financial support helps us cover our costs and to address a current shortfall in our annual operating budget. Click here to donate and thank you in advance for your contribution.
.
David R. Curry, MS
Executive Director
Center for Vaccine Ethics and Policy
Milestones :: Perspectives :: Research
Strategic Advisory Group of Experts on Immunization (SAGE) – October 2021
4 – 8 October 2021 Virtual Meeting
This meeting for the Strategic Advisory Group of Experts on Immunization (SAGE) will be held from Monday 4 to Thursday 7 October 2021 inclusive. Friday 8 October 2021 is a closed session.
Agenda and meeting information: SAGE Yellow Book for October 2021
Presentations made during the meeting will be made available online once the meeting has concluded.
SESSIONS [selected topic areas]
Global and regional reports
IA2030
Polio
COVID-19 vaccines
Malaria
Influenza
Behavioural and social drivers of vaccine uptake
Hepatitis E
Milestones :: Perspectives :: Research
Gavi Board meets to discuss routine immunisation, COVAX’s 2022 strategy
29 September 2021
:: The Gavi Board met yesterday to discuss the impact of the pandemic on routine immunisation and how COVAX can best support countries’ efforts to control the pandemic, as part of a broader consultative process to develop the 2022 COVAX strategy
:: Items covered during the meeting included the resilience of routine immunisation in Gavi-supported countries, the Vaccine Alliance’s effort to reach the estimated 12.4 million “zero-dose children” in Gavi-supported countries that do not have access to the most basic life-saving immunisation services, coverage ambitions for COVAX as well as support for delivery in COVAX AMC countries
:: José Manuel Barroso, Board Chair: “Gavi’s ambitions have never been greater than they are today: in 2022, the Vaccine Alliance will not only seek to extend its core work on routine immunisation, including reaching millions of zero-dose children, but also play a lead role in COVAX, the largest and most complex roll out of vaccines in history. This Board meeting provided an invaluable opportunity to take stock on both areas of activities as they increasingly converge on the same goal: ensuring that the most vulnerable are able to access life-saving vaccines and the benefits they bring.”
[Excerpts]
… Gavi and its Alliance partners UNICEF and WHO continue to help prepare countries for this rapid scale up. At its June meeting the Gavi Board approved new funding of US$ 799 million to support the delivery of COVAX-funded doses in lower income economies and humanitarian zones over the next two years. The funding comes in addition to a previously approved envelope of US$ 150 million in delivery support. A significant portion of this funding is being distributed rapidly and on an accelerated timeline to meet countries’ urgent vaccine rollout-related needs, for example rolling out ultra-cold chain infrastructure in time for the arrival of mRNA vaccines.
“As the global solution designed around equitable access to COVID-19 vaccines, COVAX’s strategy will continue to adapt as the pandemic evolves.” said Dr Seth Berkley, CEO of Gavi. “For many countries, COVAX is one of the primary sources of supply of COVID-19 vaccines, and we must ensure we best serve their needs. As we embark on our most intense period for deliveries yet, COVAX will also continue to focus on strengthening in-country vaccine deployment and even closer coordination with partners such as the African Union. COVAX will also need to focus further on protecting routine immunisation programmes and explore opportunities for integration with Gavi’s core strategy, with its particular focus on marginalised and missed communities”…
Gavi progress report shows resilience in childhood vaccinations despite impact of COVID-19 pandemic
29 September 2021
:: In the face of a global pandemic, routine and childhood vaccination has held relatively strong, Gavi’s latest Annual Progress Report shows, with routine immunisations dropping 4 percentage points over the course of 2020
:: While lower than 2019, vaccination rates in 2020 were characterised by a significant drop in March to May followed by a strong rebound, which is a testament to the valiant work of governments and health care workers in lower-income countries
:: Latest statistics show there are now 13.7 million “zero-dose” children in the 68 Gavi-supported countries receiving no immunisations, many of whom live in marginalised communities in remote rural areas, urban slums or conflict settings
Download the full report here
Milestones :: Perspectives :: Research
NIAID issues new awards to fund “pan-coronavirus” vaccines
September 28, 2021 — The awards are intended to fuel vaccine research for a diverse family of coronaviruses, with a primary focus on potential pandemic-causing coronaviruses, such as SARS-CoV-2.
The National Institute of Allergy and Infectious Diseases (NIAID), part of the National Institutes of Health, has awarded approximately $36.3 million to three academic institutions to conduct research to develop vaccines to protect against multiple types of coronaviruses and viral variants. The awards are intended to fuel vaccine research for a diverse family of coronaviruses, with a primary focus on potential pandemic-causing coronaviruses, such as SARS-CoV-2.
“The available COVID-19 vaccines have proven to be remarkably effective at protecting against severe disease and death,” said NIAID Director Anthony S. Fauci, M.D. “These new awards are designed to look ahead and prepare for the next generation of coronaviruses with pandemic potential.”
The new awards are funded by NIAID’s Division of Microbiology and Infectious Diseases and its Division of Allergy, Immunology, and Transplantation through the Emergency Awards Notice of Special Interest (NOSI) on Pan-Coronavirus Vaccine Development Program Projects. The notice was issued in November 2020 while many SARS-CoV-2 vaccines were still under development because a critical need remained for prophylactic vaccines offering broad protective immunity against other coronaviruses, such as Middle East Respiratory Syndrome Coronavirus (MERS-CoV) and Severe Acute Respiratory Syndrome Coronavirus (SARS-CoV).
The awards are designed to fund multidisciplinary teams at each institution to conduct research focused on incorporating understanding of coronavirus virology and immunology, immunogen design, and innovative vaccine and adjuvant platforms and technologies to discover, design, and develop pan-coronavirus vaccine candidates that provide broad protective immunity to multiple coronavirus strains. Specific programs will address coronavirus diversity and infectious potential in humans, include innovative immunogen design and vaccine platforms, and approaches to elicit potent and durable pan-coronavirus immunity, and evaluate vaccine candidates in preclinical models. The awardees are expected to be flexible in the response to emerging knowledge about SARS-CoV-2 immune responses and infection and factor in new information as vaccines candidates are developed. Additional awards are expected to be issued under the NOSI in 2022 to support pan-coronavirus vaccine research at more institutions…
A key goal of the initiative is to develop multivalent vaccine platforms and strategies suitable for use in vulnerable populations and to understand vaccine-induced responses and efficacy related to a person’s age or sex.
Milestones :: Perspectives :: Research
::::::
COVID – PHEIC
Editor’s Note:
As is obvious to all, the sheer volume of strategic announcements, regulatory actions, country program decisions, commentary, and, indeed, misinformation around COVID response continues at extraordinary levels. Our weekly digest strives to present a coherent and comprehensive snapshot, but cannot be exhaustive, If you recognize a missed strategic development, a new source of rigorous analysis, or an insight/commentary that would benefit our common understanding, please advise me…we will review all suggestions and consider them for inclusion in a subsequent edition: david.r.curry@ge2p2global.org
We are seeking access to modelling which engages scenarios and articulates imperatives around a COVID-19 pandemic end-game through at least a 2025 horizon. We assess that WHO must be conducting or contracting for such modeling – or should recognize an imperative to be doing so in its global health governance role. If we have missed such modeling in progress, we would be delighted to be advised of it and will include it in our coverage.
::::::
COVID Vaccines – OCHA:: HDX
COVID-19 Data Explorer: Global Humanitarian Operations
COVID-19 Vaccine Roll-out
Oct 1, 2021 | COVAX (WHO,GAVI,CEPI), UNDESA, Press Reports | DATA
Global COVID-19 Figures: 233M total confirmed cases; 4.8M total confirmed deaths
Global vaccines administered: 6.27B
Number of Countries: 29 [29 week ago]
COVAX Allocations Round 4-6 (Number of Doses): 120M [120M week ago]
COVAX Delivered (Number of Doses): 95M [93M week ago]
Other Delivered (Number of Doses): 140M [140M week ago]
Total Delivered (Number of Doses): 240M [230M week ago]
Total Administered (Number of Doses): 220M [210M week ago]
Milestones :: Perspectives :: Research
World Bank Vaccine Operations Portal
https://www.worldbank.org/en/who-we-are/news/coronavirus-covid19/world-bank-support-for-country-access-to-covid-19-vaccines
As of September 30, 2021, the World Bank approved operations to support vaccine rollout in 61 countries amounting to $5.8 billion. See the latest project financing, project documents and procurement information in the list below. More information will be shared here as it becomes available.
Milestones :: Perspectives :: Research
Multilateral Leaders Task Force on COVID-19 [IMF, World Bank Group, WHO, WTO]
A joint initiative from the International Monetary Fund, World Bank Group, World Health Organization, and World Trade Organization to accelerate access to COVID-19 vaccines, therapeutics and diagnostics by leveraging multilateral finance and trade solutions, particularly in low- and middle-income countries. Website accessed 2 Oct 2021: https://data.covid19taskforce.com/data The global view below is complemented by country-specific dashboards here.
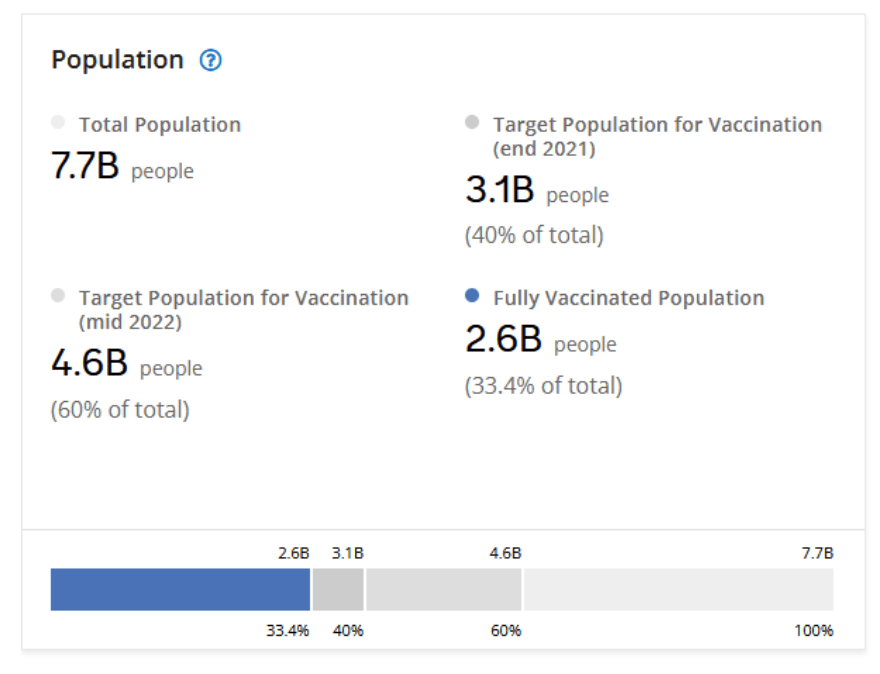
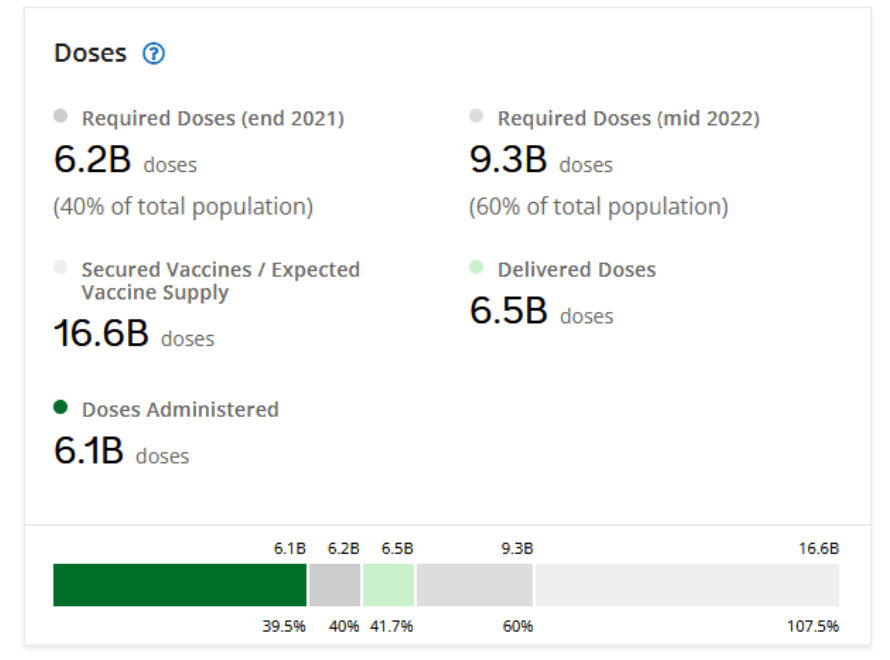
Milestones :: Perspectives :: Research
::::::
Coronavirus [COVID-19] – WHO
Public Health Emergency of International Concern (PHEIC)
https://www.who.int/emergencies/diseases/novel-coronavirus-2019
Weekly Epidemiological and Operational updates
Last update: 2 Oct 2021
Confirmed cases :: 233 503 524 [230 418 451 week ago]
Confirmed deaths :: 4 777 503 [4 724 876 week ago]
Vaccine doses administered: 6 143 369 655 [5 874 934 542 week ago]
:::::::
Weekly epidemiological update on COVID-19 – 28 September 2021
Overview
Globally, the numbers of weekly COVID-19 cases and deaths continued to decline. Over 3.3 million new cases and over 55 000 new deaths were reported during the week of 20 – 26 September 2021, decreases of 10% as compared to the previous week for both cases and deaths. The largest decrease in new weekly cases was reported from the Eastern Mediterranean Region (17%), followed by the Western Pacific Region (15%), the Region of the Americas (14%), the African Region (12%) and the South-East Asia Region (10%); while weekly cases in the European Region were similar to the previous week. The cumulative number of confirmed cases reported globally is now over 231 million and the cumulative number of deaths is more than 4.7 million.
In this edition, two special focus updates are provided on:
:: Approaches to determining waning COVID-19 vaccine effectiveness
:: SARS-CoV-2 Variants of Concern (VOCs) Alpha, Beta, Gamma and Delta which includes updates on the geographic distribution of VOCs
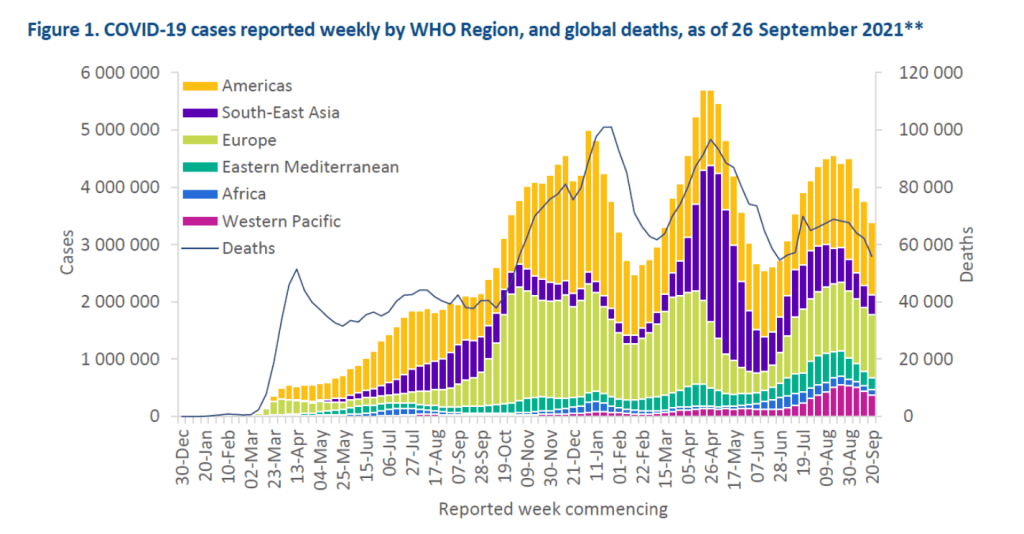
Summary of the COVID-19 Weekly Operational Update
The Weekly Operational Update (WOU) is a report provided by the COVID-19 Strategic Preparedness and Response Plan (SPRP) monitoring and evaluation team which aims to update on the ongoing global progress against the COVID-19 SPRP 2021 framework.
In this week’s edition of the COVID-19 Weekly Operational Update, published on 28 September, highlights of country-level actions and WHO support to countries include:
Delivering 2 million syringes for Sri Lanka’s COVID-19 vaccination drive
Shipment of WHO life-saving medical supplies to Kabul, Afghanistan with support from Qatar
WHO logistics hub airlifts largest single shipment of humanitarian cargo to Ethiopia
WHO/Europe and Germany support children with disabilities in Belarus
Rebooting COVID-19 response strategy and measures in Cambodia
Expanding capacity for Integrated Disease Surveillance and Response (IDSR) in the African Region
External Quality Assessment for laboratories testing for SARS-CoV-2
Testing Rapid Response Mobile Laboratories (RRML) deployment procedures and minimum standards in first virtual tabletop (V-TTX) exercise for RRML/GOARN
Connecting countries to share experiences and learnings from their COVID-19 vaccine roll-out using the mini-cPIE (COVID-19 vaccination Intra-Action Review) process
Progress on a subset of indicators from the SPRP 2021 Monitoring and Evaluation Framework
Updates on WHO’s financing to support countries in SPRP 2021 implementation and provision of critical supplies.
Milestones :: Perspectives :: Research
Status of COVID-19 Vaccines within WHO EUL/PQ evaluation process 19 August 2021
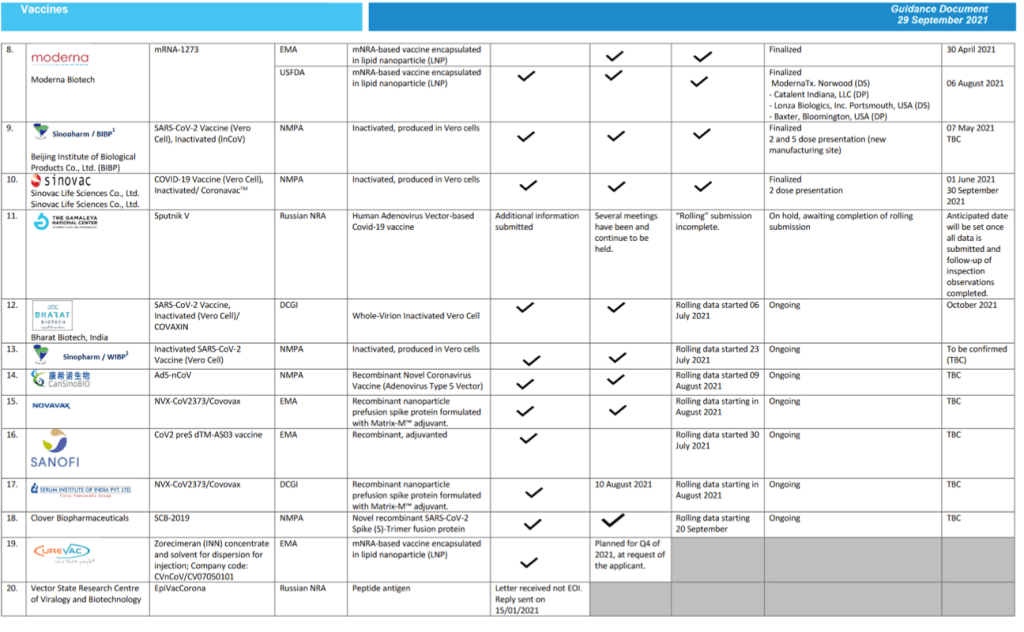
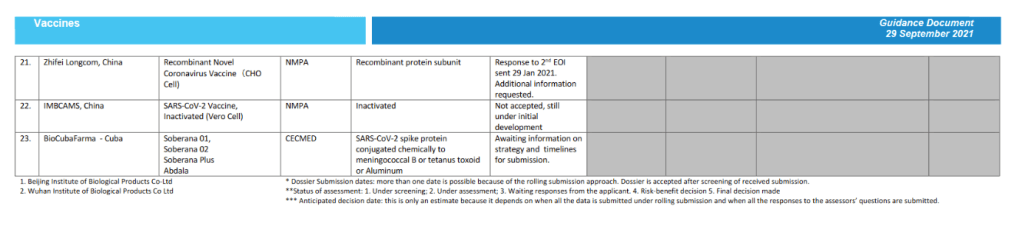
For 23 vaccine candidates, presents Manufacturer, Name of Vaccine, NRA of Record, Platform, EOI Accepted Status, Pre-submission Meeting Held Status, Dossier Accepted for Review, Status of Assessment; Anticipated/Completed Decision Date
[Full scale view available at title link above]
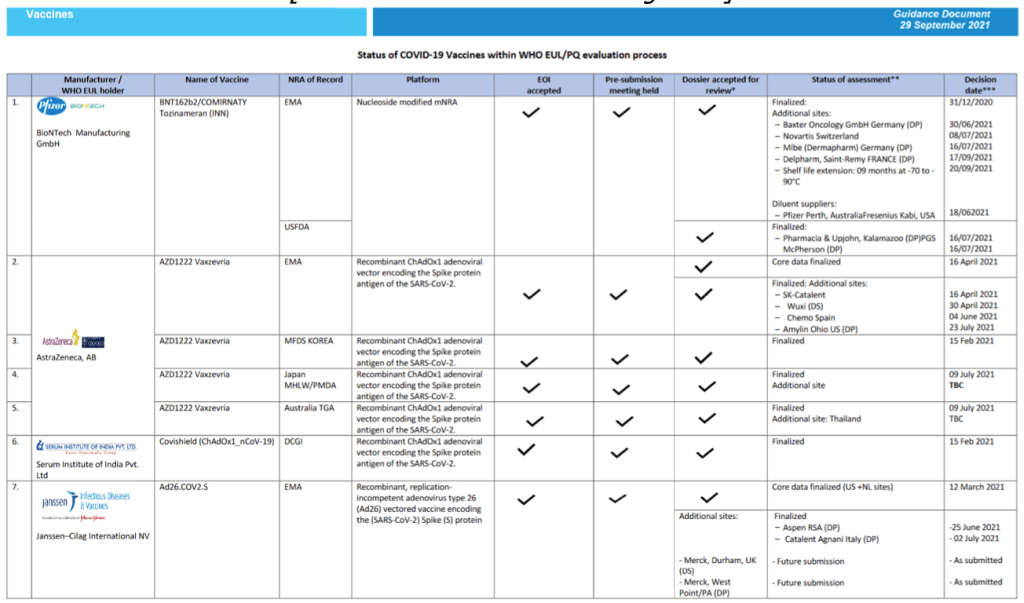
Milestones :: Perspectives :: Research
COVID Vaccine Developer/Manufacturer Announcements
[relevant press releases/announcement from organizations from WHO EUL/PQ listing above]
AstraZeneca
Press Releases – No new digest announcements identified
BioCubaFarma – Cuba
Últimas Noticias – [Website not responding at inquiry; receiving 403-Forbidden]
CanSinoBIO
News – [Website not responding at inquiry]
Clover Biopharmaceuticals – China
News – No new digest announcements identified
Curevac [Bayer Ag – Germany]
News – No new digest announcements identified
Gamaleya National Center
Latest News and Events – No new digest announcements identified [See Russia/RFID below]
IMBCAMS, China
Home – No new digest announcements identified
Janssen/JNJ
Press Releases
Sep 29, 2021 United States
Janssen Announces Start of Phase 3 Trial for Investigational Respiratory Syncytial Virus (RSV) Vaccine in Older Adults
Positive Phase 2b data supporting further evaluation will be presented at IDWeek 2021
Moderna
Press Releases
October 1, 2021
Joint Statement from Moderna and Takeda
STATEMENT REGARDING Moderna COVID-19 Vaccine Recall Investigation Report – October 2021
Takeda and Moderna today published a report of the investigation prompted by the observation of foreign particles in unpunctured vials from a single lot of Moderna’s COVID-19 vaccine distributed in Japan by Takeda. The lot was suspended on August 26, 2021, JST and voluntarily recalled on September 2, 2021, JST. Two other lots manufactured in the same series were included in the suspension and voluntary recall as a precautionary measure…
September 30, 2021
Moderna to Invest in New Science Center in Cambridge, MA
462,000 square foot state-of-the-art building targeting LEED Zero certification and designed to be the most sustainable commercial lab building in Cambridge
CAMBRIDGE, Mass.–(BUSINESS WIRE)–Sep. 30, 2021– Moderna, Inc. (Nasdaq: MRNA), a biotechnology company pioneering messenger RNA (mRNA) therapeutics and vaccines, today announced it is investing in a new science center, known as the Moderna Science Center, at 325 Binney Street in Cambridge, Massachusetts to create a purpose-built space to support the Company’s next chapter of discovery….
Novavax
Press Releases – No new digest announcements identified
Pfizer
Recent Press Releases
09.29.2021
Positive Top-line Results of Pfizer’s Phase 3 Study Exploring Coadministration of Prevnar 20™ With Seasonal Flu Vaccine in Older Adults Released
09.28.2021
Pfizer and BioNTech Submit Initial Data to U.S. FDA From Pivotal Trial of COVID-19 Vaccine in Children 5 to <12 Years of Age
Formal submission to request Emergency Use Authorization to follow in the coming weeks
…The Companies announced positive topline results from the pivotal trial on September 20, 2021. In the trial, which included 2,268 participants 5 to <12 years of age, the vaccine demonstrated a favorable safety profile and elicited robust neutralizing antibody responses using a two-dose regimen of 10 μg doses… 09.27.2021 Pfizer Starts Study of mRNA-Based Next Generation Flu Vaccine Program
Sanofi Pasteur
Press Releases
September 28 2021
Press releases
Sanofi to focus its COVID-19 development efforts on the recombinant vaccine candidate
Recent positive interim results of Sanofi’s mRNA-based COVID-19 vaccine candidate Phase 1/2 study confirm the company’s platform robust capabilities and strategy in mRNA.
Taking into account public health needs and given sufficient mRNA COVID-19 vaccines supply can be expected going forward, Sanofi has decided not to pursue the development of its COVID-19 mRNA candidate into a Phase 3 clinical study and will focus on completing the final development steps of its COVID-19 recombinant vaccine, developed in partnership with GSK.
Building on its positive results, the company will focus its mRNA resources in its newly created mRNA Center of Excellence to address future pandemics and other infectious diseases and therapeutics where there is a strong unmet need.
September 28 2021
Press releases
Sanofi announces positive Phase 1/2 study interim results for its first mRNA-based vaccine candidate
:: High seroconversion across the three dosages tested and comparable tolerability to other unmodified mRNA COVID-19 vaccines
:: Now accelerating transformation of acquired platform to modified mRNA and targeting a modified quadrivalent flu mRNA vaccine in the clinic in 2022
September 27 2021
Press releases
New research presented at IDWeek 2021 reinforces Sanofi’s robust vaccines pipeline and commitment to advancing public health protection
Enrollment begins for Sanofi and GSK’s Phase 3 efficacy trial of COVID-19 vaccine candidate in Nepal, led by IVI
Sept. 28, 2021
Serum Institute of India
NEWS & ANNOUNCEMENTS – No new digest announcements identified
Sinopharm/WIBPBIBP
News – No new digest announcements identified
Sinovac
Press Releases – No new digest announcements identified
Vector State Research Centre of Viralogy and Biotechnology
Home – No new digest announcements identified
Zhifei Longcom, China
[Anhui Zhifei Longcom Biologic Pharmacy Co., Ltd.]
[No website identified]
::::::
GSK
Press releases for media
28 September 2021 FDA grants Priority Review to ViiV Healthcare’s New Drug Application for cabotegravir long-acting for prevention of HIV
Enrollment begins for Sanofi and GSK’s Phase 3 efficacy trial of COVID-19 vaccine candidate in Nepal, led by IVI
Sept. 28, 2021
…International Vaccine Institute (IVI) will lead the clinical trial in Nepal to assess the safety, efficacy and immunogenicity of an adjuvanted recombinant-protein COVID-19 vaccine candidate
Phase 3 international clinical trial includes volunteers from several countries, including sites in the US, Asia, Africa and Latin America…
Merck
News releases
Merck and Ridgeback’s Investigational Oral Antiviral Molnupiravir Reduced the Risk of Hospitalization or Death by Approximately 50 Percent Compared to Placebo for Patients with Mild or Moderate COVID-19 in Positive Interim Analysis of Phase 3 Study
:: At the Interim Analysis, 7.3 Percent of Patients Who Received Molnupiravir Were Hospitalized Through Day 29, Compared With 14.1 Percent of Placebo-Treated Patients Who were Hospitalized or Died
:: Merck Plans to Seek Emergency Use Authorization in the U.S. as Soon as Possible and to Submit Applications to Regulatory Agencies Worldwide
:: If Authorized, Molnupiravir Could be the First Oral Antiviral Medicine for COVID-19
October 1, 2021
SK Biosciences
Press releases – No new digest announcements identified
Milestones :: Perspectives :: Research
UNICEF COVID-19 Vaccine Market Dashboard :: Agreements Table Accessed 2 Oct 2021
An overview of information collected from publicly announced bilateral and multilateral supply agreements [agreements view since last week’s edition]
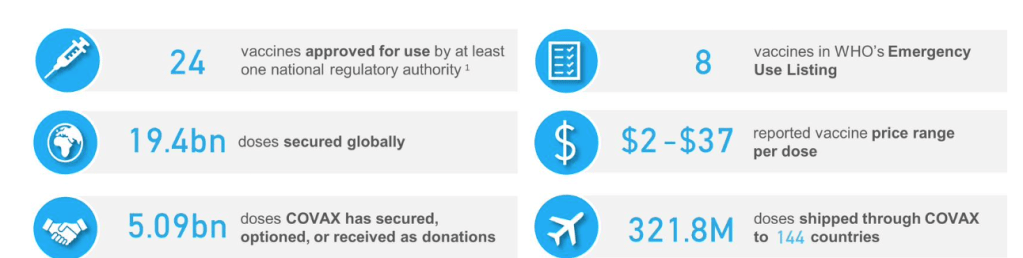
No new bilateral deals reported since 17 Sep 2021.
Milestones :: Perspectives :: Research
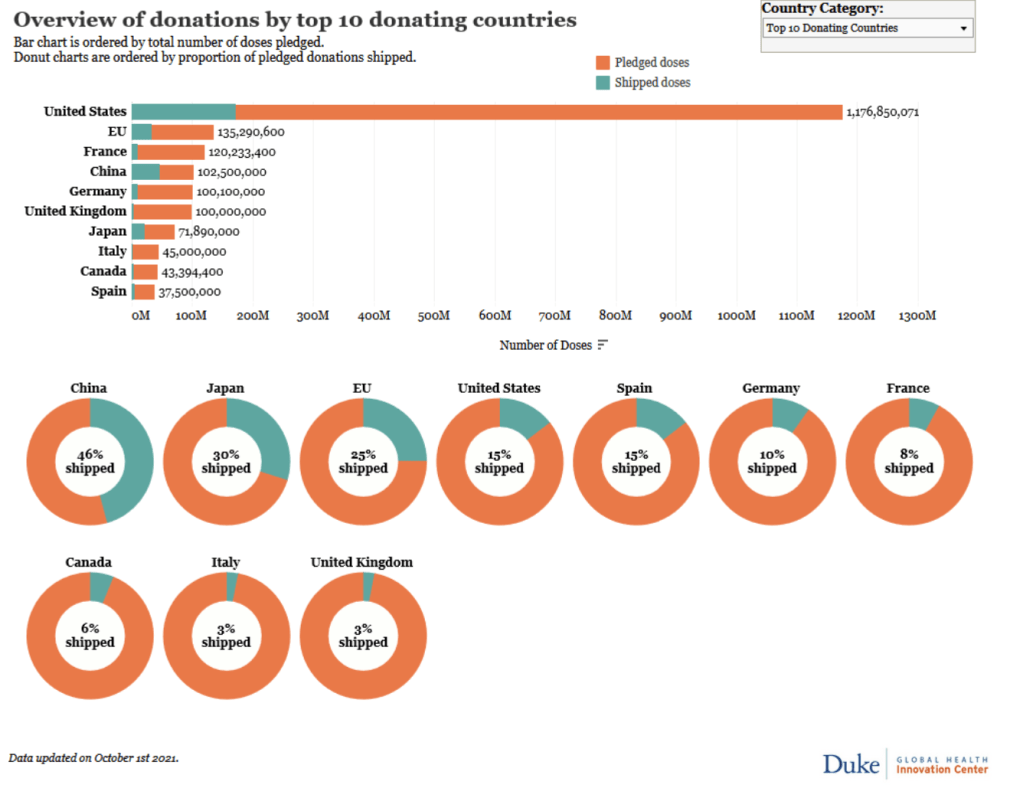
Duke – Launch and Scale Speedometer
The Race for Global COVID-19 Vaccine Equity
A flurry of nearly 200 COVID-19 vaccine candidates are moving forward through the development and clinical trials processes at unprecedented speed; more than ten candidates are already in Phase 3 large-scale trials and several have received emergency or limited authorization. Our team has aggregated and analyzed publicly available data to track the flow of procurement and manufacturing and better understand global equity challenges. We developed a data framework of relevant variables and conducted desk research of publicly available information to identify COVID vaccine candidates and status, deals and ongoing negotiations for procurement and manufacturing, COVID burden by country, and allocation and distribution plans. We have also conducted interviews with public officials in key countries to better understand the context and challenges facing vaccine allocation and distribution
[accessed 24 July 2021]
See our COVID Vaccine Purchases research
See our COVID Vaccine Manufacturing research
See our COVID Vaccine Donations & Exports research
Milestones :: Perspectives :: Research
Global Dashboard on COVID-19 Vaccine Equity
The Dashboard is a joint initiative of UNDP, WHO and the University of Oxford with cooperation across the UN system, anchored in the SDG 3 Global Action Plan for Healthy Lives and Well-being for All.
Dashboard on Vaccine Equity [accessed 2 Oct 2021]: https://data.undp.org/vaccine-equity/
See also visualization on Vaccine Access and Vaccine Affordability
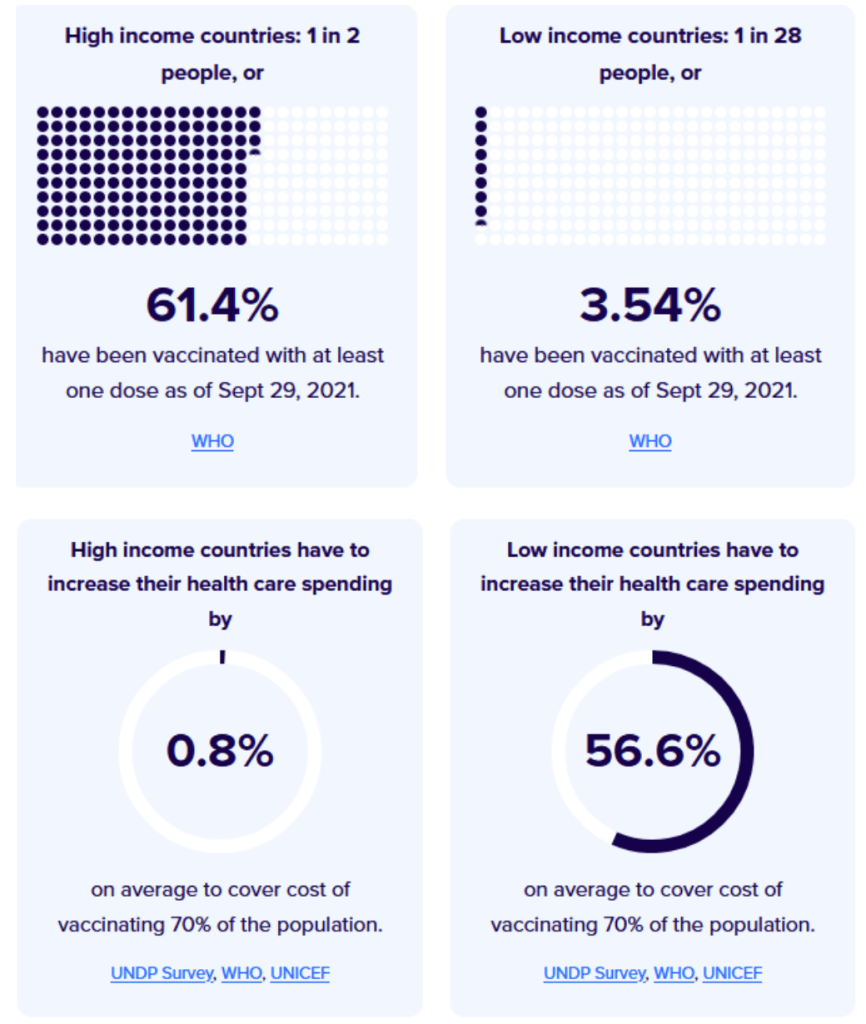
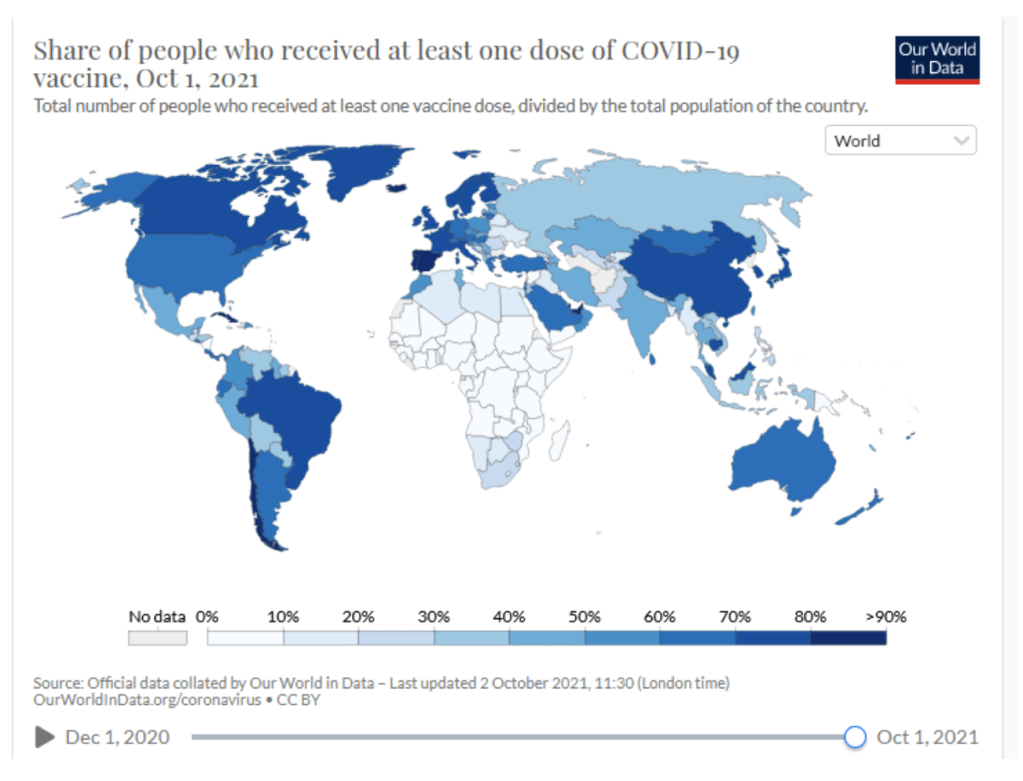
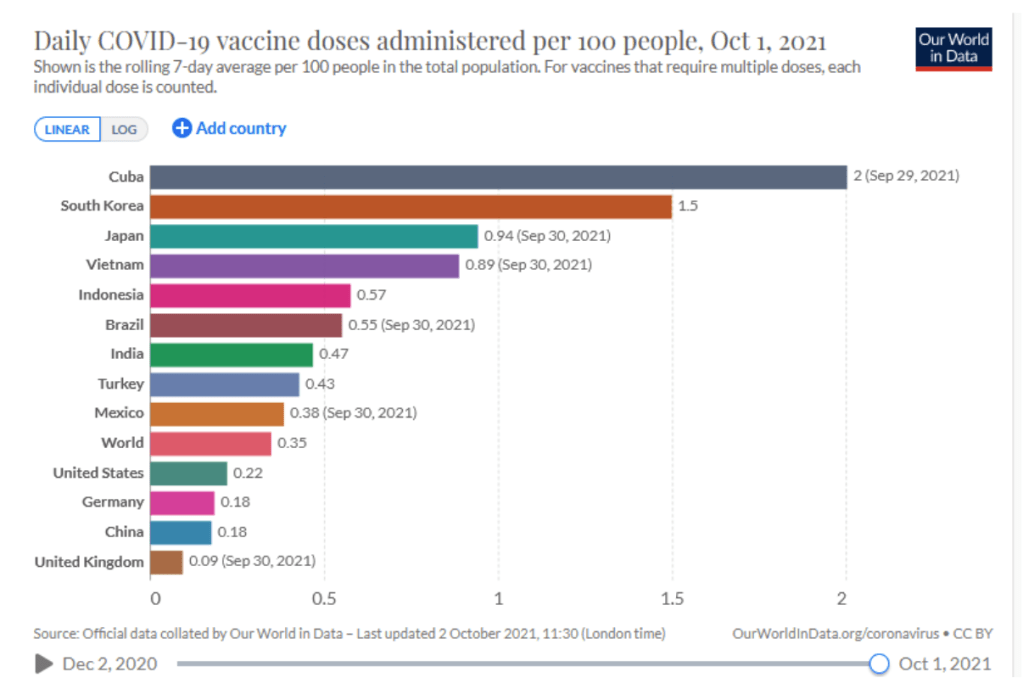
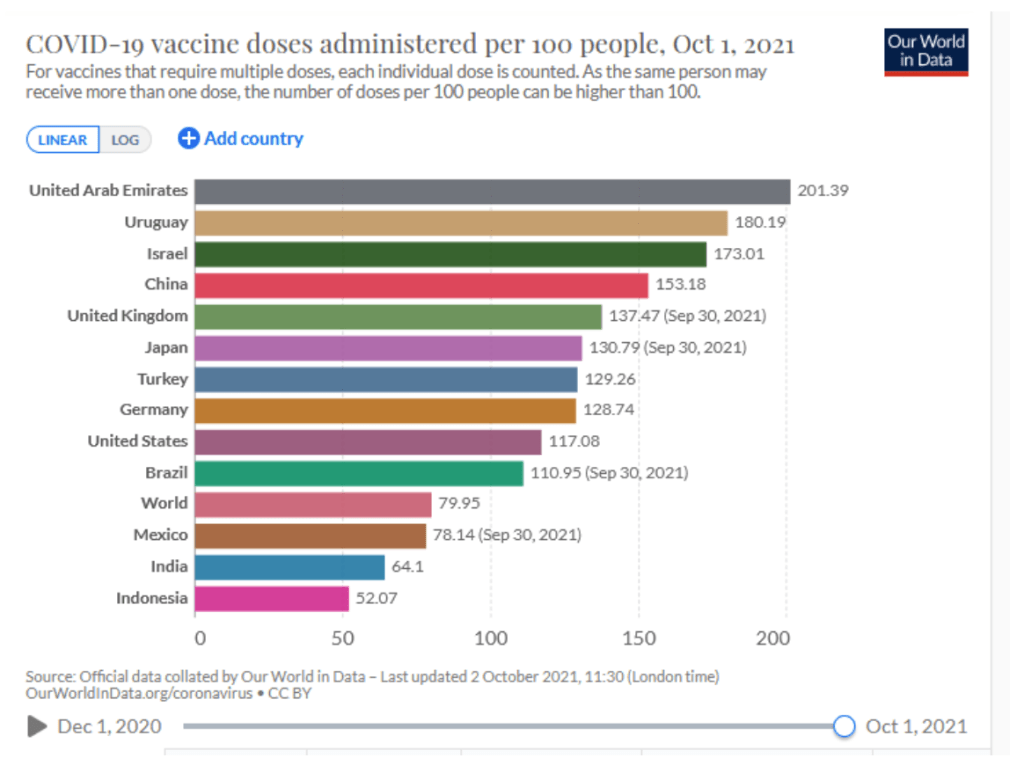
Milestones :: Perspectives :: Research
Our World in Data
Coronavirus (COVID-19) Vaccinations [Accessed 2 Oct 2021]
:: 45.5% of the world population has received at least one dose of a COVID-19 vaccine.
:: 6.3 billion doses have been administered globally, and 27.33 million are now administered each day.
:: Only 2.3% of people in low-income countries have received at least one dose.
Milestones :: Perspectives :: Research
U.S.: COVID-19 Vaccines – Announcements/Regulatory Actions/Deployment
::::::
Vaccines and Related Biological Products Advisory Committee– FDA
https://www.fda.gov/advisory-committees/blood-vaccines-and-other-biologics/vaccines-and-related-biological-products-advisory-committee
Vaccines and Related Biological Products Advisory Committee October 14-15, 2021 Meeting Announcement
Agenda
…Under Topic 1, the committee will meet in open session to discuss the Emergency Use Authorization (EUA) of the ModernaTX Inc. COVID-19 vaccine for the administration of an additional dose, or “booster” dose, following completion of the primary series, to individuals 18 years of age and older. On October 15, 2021, under Topic II, the committee will meet in open session to discuss the Emergency Use Authorization (EUA) of the Janssen Biotech Inc. COVID-19 vaccine for the administration of an additional dose, or “booster” dose, to individuals 18 years of age and older.
::::::
White House [U.S.]
Briefing Room – Selected Major COVID Announcements
Statement by President Joe Biden on 700,000 American Deaths from COVID-19
October 02, 2021 • Statements and Releases
Press Briefing by White House COVID-19 Response Team and Public Health Officials
October 01, 2021 • Press Briefings
Press Briefing by White House COVID-19 Response Team and Public Health Officials
September 28, 2021 • Press Briefings
President Biden Announces Intent to Nominate Dr. John N. Nkengasong as Ambassador-at-Large and Coordinator of United States Government Activities to Combat HIV/AIDS Globally
September 27, 2021 • Statements and Releases
Remarks by President Biden While Receiving a COVID-19 Booster Shot
September 27, 2021 • Speeches and Remarks
Milestones :: Perspectives :: Research
Europe: COVID-19 Vaccines – Announcements/Regulatory Actions/Deployment
European Medicines Agency
News & Press Releases
News: EMA welcomes new Head of International Affairs (new)
Last updated: 01/10/2021
EMA is pleased to announce the appointment of Martin Harvey Allchurch as its new head of International Affairs as of 1 October 2021.
Martin Harvey Allchurch brings a wealth of EMA and international experience to this role, having worked in a number of management positions at the Agency, as well as at Unitaid, the World Health Organisation-hosted partnership for access to innovative healthcare in low- and middle-income countries…
News: Meeting highlights from the Pharmacovigilance Risk Assessment Committee (PRAC) 27-30 September 2021 (new)
PRAC, Last updated: 01/10/2021
…PRAC assessment of rare cases of venous thromboembolism with COVID-19 Vaccine Janssen
The PRAC has concluded that there is a possible link to rare cases of venous thromboembolism (VTE) with COVID-19 Vaccine Janssen…
…The PRAC has now reviewed new evidence from the study described above, as well as new evidence from another large clinical study. In this second study, there was no increase in venous thromboembolic events among individuals who received COVID-19 Vaccine Janssen. The PRAC also reviewed evidence from the post marketing setting – that is data gathered while the vaccine is used in the context of vaccination campaigns. When taking all evidence into account, the committee concluded that there is a reasonable possibility that rare cases of VTE are linked to vaccination with COVID-19 Vaccine Janssen.
The committee is therefore recommending listing VTE as a rare side effect of COVID-19 Vaccine Janssen in the product information, together with a warning to raise awareness among healthcare professionals and people taking the vaccine, especially those who may have an increased risk of VTE…
News: Transatlantic Taskforce continues international fight against antimicrobial resistance (new)
Last updated: 30/09/2021
News: EMA implements new measures to minimise animal testing during medicines development (new)
Last updated: 29/09/2021
::::::
European Centre for Disease Prevention and Control
https://www.ecdc.europa.eu/en
Latest Updates
Publication
Rapid Risk Assessment: Assessing SARS-CoV-2 circulation, variants of concern, non-pharmaceutical interventions and vaccine rollout in the EU/EEA, 16th update
Risk assessment
30 Sep 2021
Since its emergence in March 2021, the B.1.617.2 (Delta) variant of concern (VOC) has rapidly become predominant across the European Union/European Economic Area (EU/EEA). More than 99% of newly reported cases are attributed to this variant. The Delta variant has demonstrated a significant transmission advantage relative to previously circulating SARS-CoV-2 strains. However, full vaccination remains protective against severe outcomes such as hospitalisation, admission to intensive care and death. Currently available vaccines have played a crucial role in limiting viral circulation and in particular, limiting the impact of infections by the Delta variant.
Despite the fact that over 565 million vaccine doses have been administered in the EU/EEA so far, only 61.1% (range: 18.4–79.4%) of the total population in the EU/EEA have been fully vaccinated to date. The total population includes children and adolescents for whom the vaccine is not available or who may not be included in national target groups yet. There is considerable inter-country and sub-national variation in vaccine uptake, resulting in large proportions of the EU/EEA population remaining susceptible to SARS-CoV-2 infection.
Modelling scenarios that consider vaccination coverage, vaccine effectiveness, natural immunity and population contact rates—in the context of continued Delta circulation—indicate that the potential burden of disease risk in the EU/EEA from the Delta variant is high between now to the end of November, unless vaccination coverage can be increased rapidly in the total population in the next few weeks.
News
High risk of autumn surge in COVID-19 cases and deaths in countries with insufficient vaccination coverage, warns ECDC
News story – 30 Sep 2021
EU/EEA countries that have not yet achieved high enough COVID-19 vaccination coverage in their total populations, which are planning to relax non-pharmaceutical interventions during the next two weeks, run a high risk of experiencing a significant surge of cases, hospitalisations and mortality from now until the end of November. This is indicated by a new SARS-CoV-2 Rapid Risk Assessment published today by the European Centre for Disease Prevention and Control (ECDC).
::::::
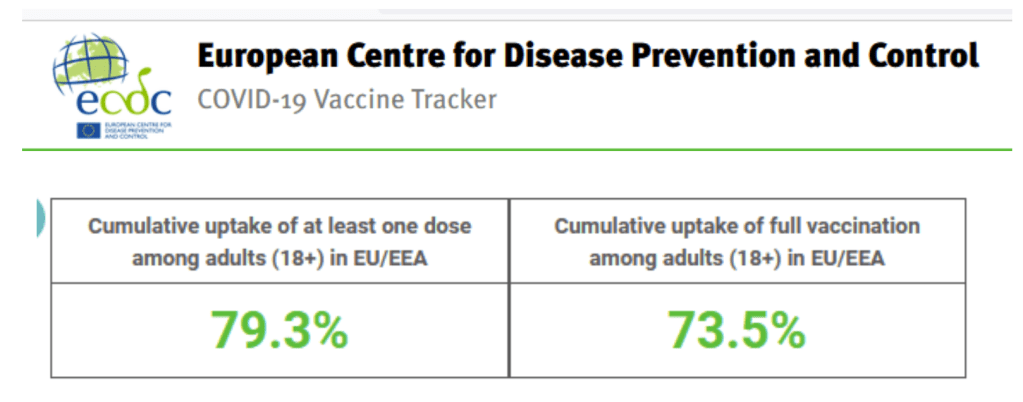
Accessed 2 Oct 2021
https://vaccinetracker.ecdc.europa.eu/public/extensions/COVID-19/vaccine-tracker.html#uptake-tab
::::::
European Commission
https://ec.europa.eu/commission/presscorner/home/en
Press release 2 October 2021
EU Humanitarian Air Bridge delivers life-saving medical aid to Afghanistan
Press release 1 October 2021
Code of Practice on disinformation: Commission welcomes new prospective signatories and calls for strong and timely revision
The topic of disinformation remains high on the Commission’s agenda. Eight new prospective signatories joined the revision process of the Code of Practice on disinformation during the latest signatories Assembly meeting yesterday.
Press release 28 September 2021
European Health Union: Towards a reform of EU’s pharmaceutical legislation
Today, as part of its work to create a future-proof and crisis-resilient regulatory framework for the pharmaceutical sector, the Commission has published a public consultation on the revision of the EU’s pharmaceutical legislation.
Milestones :: Perspectives :: Research
Africa: COVID-19 – Announcements/Regulatory Actions/Deployment

Accesses 2 Oct 2021. Full scale, interactive dashboard available at:
https://africacdc.org/covid-19-vaccination/
Fifteen African countries hit 10% COVID-19 vaccination goal
30 September 2021 WHO AFRO
Brazzaville – Fifteen African countries—nearly a third of the continent’s 54 nations—have fully vaccinated 10% of their people against COVID-19.
The global goal of fully vaccinating 10% of every country’s population by 30 September was set in May by the World Health Assembly, the world’s highest health policy-setting body. Almost 90% of high income-countries have met this target.
Seychelles and Mauritius have fully vaccinated over 60% of their populations, Morocco 48% and Tunisia, Comoros and Cape Verde over 20%. Most of the African countries that have met the goal have relatively small populations and 40% are small island developing states.
All these countries have enjoyed sufficient supplies of vaccines, and many could access doses from separate sources in addition to those delivered through the COVAX Facility, the global platform to ensure equitable access to vaccines. Half of the 52 African countries that have received COVID-19 vaccines have fully vaccinated just 2% or less of their populations.
“The latest data shows modest gains but there is still a long way to go to reach the WHO target of fully vaccinating 40% of the population by the end of the year. Shipments are increasing but opaque delivery plans are still the number one nuisance that hold Africa back,” said Dr Richard Mihigo, Immunization and Vaccines Development Programme Coordinator for the World Health Organization (WHO) Regional Office for Africa…
Milestones :: Perspectives :: Research
Russia: COVID-19 Vaccines – Announcements/Regulatory Actions/Deployment
Russia: Sputnik V – “the first registered COVID-19 vaccine”
https://sputnikvaccine.com/newsroom/pressreleases/
Press Releases
A clinical study of the combination of AstraZeneca and Sputnik Light vaccines in Azerbaijan shows strong neutralizing antibodies growth in the majority of participants
Press release, 27.09.2021
Milestones :: Perspectives :: Research
India: COVID-19 Vaccines – Announcements/Regulatory Actions/Deployment
Ministry of Health and Family Welfare
https://www.mohfw.gov.in/
Latest Updates
29.09.2021
National Guidelines for Safe Dental Practice During Covid-19 pandemic
Government of India – Press Information Bureau
Latest Press Releases
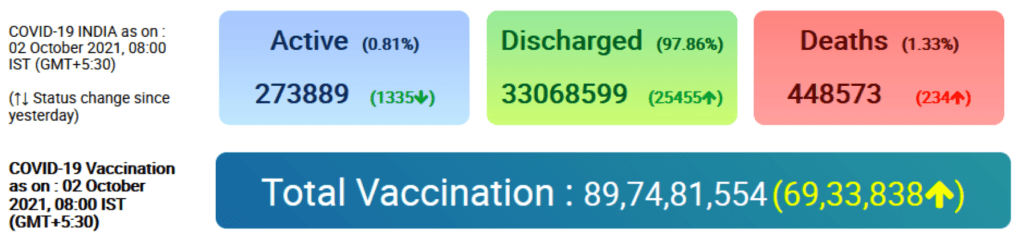
COVID-19 Vaccination Update – Day 260
India’s cumulative vaccination coverage crosses 90 crore landmark milestone
More than 65 lakh Vaccine doses administered today till 7 pm
Posted On: 02 OCT 2021 9:30PM by PIB Delhi
India’s COVID-19 vaccination coverage has crossed 90 Crore landmark milestone (90,42,59,810) today. More than 65 lakh (65,27,196) Vaccine Doses have been administered till 7 pm today. The daily vaccination tally is expected to increase with the compilation of the final reports for the day by late tonight.
Indian Council for Medical Research (ICMR)
https://www.icmr.gov.in/media.html
Press Releases
No new digest content identified
Milestones :: Perspectives :: Research
China: COVID-19 Vaccines – Announcements/Regulatory Actions/Deployment
No new digest content identified. See China CDC below for additional announcements.
Emergencies
POLIO
Public Health Emergency of International Concern (PHEIC)
https://polioeradication.org/polio-today/polio-now/this-week/
Polio this week as of 29 September 2021
:: The September edition of Polio News with the latest news, programme and case updates is now available here.
Summary of new WPV and cVDPV viruses this week (AFP cases and ES positives):
:: Guinea: one cVDPV2 positive environmental sample
:: Madagascar: two cVDPV1 cases
:: Mauritania: two cVDPV2 positive environmental samples
:: Senegal: one cVDPV2 case and three cVDPV2 positive environmental samples
::::::
Event – Eradicating Polio: What more Is Needed?
WFPHA – World Federation of Public Health Associations
Sep 22, 2021
Polio cases have fallen 99.9% since 1988. In 2020 Africa was certified polio free by the World Health Organization. However, polio will remain a key public health concern until such time as there are no wild… On October 12, 2021, at 10:00 – 11:00 (CEST), “Eradicating Polio: What more Is Needed?” webinar will be held to focus on the barriers, challenges and leverages to reach every child and eradicate polio globally. Register
::::::
::::::
WHO/OCHA Emergencies
Editor’s Note:
WHO has apparently reorganized and fundamentally shifted how it judges and tracks “emergencies”. We found no announcement of descriptive information to share and present the webpage structure as encountered below. Obviously, the dates associated with some of these emergencies suggest that this is an archival platform as well as a current emergencies resource.
Health emergencies list – WHO
“The health emergencies list details the disease outbreaks, disasters and humanitarian crises where WHO plays an essential role in supporting countries to respond to and recover from emergencies with public health consequences.”
Crisis in Tigray, Ethiopia [Last apparent update: 5 Aug 2021]
Ebola outbreak, Democratic Republic of the Congo, 2021 [Last apparent update: 17 Aug 2021]
Ebola outbreak outbreak, N’Zerekore, Guinea, 2021 [Last apparent update: 17 Aug 2021]
Coronavirus disease (COVID-19) pandemic [See COVID above]
Ebola outbreak, Equateur Province, Democratic Republic of the Congo, 2020
[Last apparent update: 17 Aug 2021]
Ebola outbreak, North Kivu, Ituri, Democratic Republic of the Congo, 2018 – 2020
[Last apparent update: 17 Aug 2021]
Ebola outbreak, Democratic Republic of the Congo, 2018 [Last apparent update: 24 July 2018]
Yemen crisis [Last apparent update: 12 February 2021]
Syria crisis [Last apparent update: 18 June 2021]
Somalia crisis [Last apparent update: 24 March 2018]
Nigeria crisis [Last apparent update: 9 May 2018]
Ebola outbreak, Democratic Republic of the Congo, 2017 [Last apparent update: 17 Aug 2021]
Zika virus disease outbreak, 2015-2016 [Last apparent update: 24 Jan 2020]
Ebola outbreak: West Africa, 2014-2016 [Last apparent update: 17 Aug 2021]
Iraq crisis [Last apparent update: 9 Jan 2008]
South Sudan crisis [Last apparent update: 23 Sep 2020]
Avian influenza A (H7N9) virus outbreak [Last apparent update: 13 September 2021]
Middle East respiratory syndrome (MERS-CoV) outbreak [Last apparent update: 8 July 2019]
Influenza A (H1N1) virus, 2009-2010 pandemic [Last apparent update: 10 Aug 2010]
::::::
UN OCHA – Current Emergencies
Current Corporate Emergencies
Afghanistan
No new digest content identified
Northern Ethiopia
Ethiopia – Northern Ethiopia Humanitarian Update Situation Report, 30 Sept 2021
HIGHLIGHTS
Malnutrition among pregnant and lactating women screened during the week is unprecedently high with 79% of some 15,000 women diagnosed with acute malnutrition in Tigray.
Huge price hike due to severe shortages of essential commodities in Tigray.
Some 126,000 people received food (half the number of people reached the prior week), of whom 52% received only 2 kg of pulses due to reduced supplies.
Between 23 and 25 September, 33 trucks with food commodities entered Tigray, and 35 empty trucks returned to Semera from Mekelle to carry more food stock back into the region.
Humanitarian partners continue to scale up response in Afar and Amhara regions.
::::::
::::::