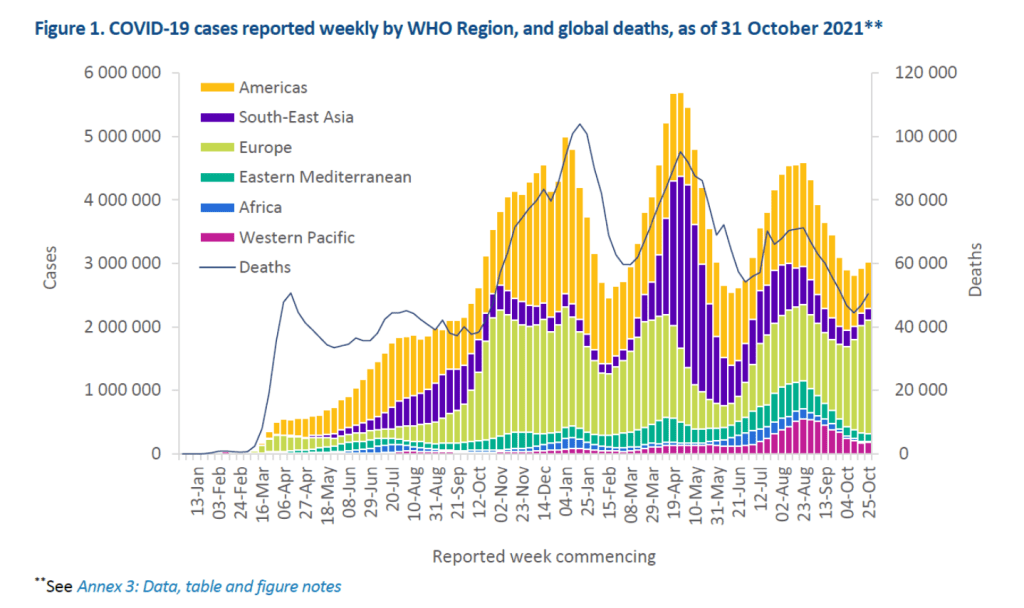
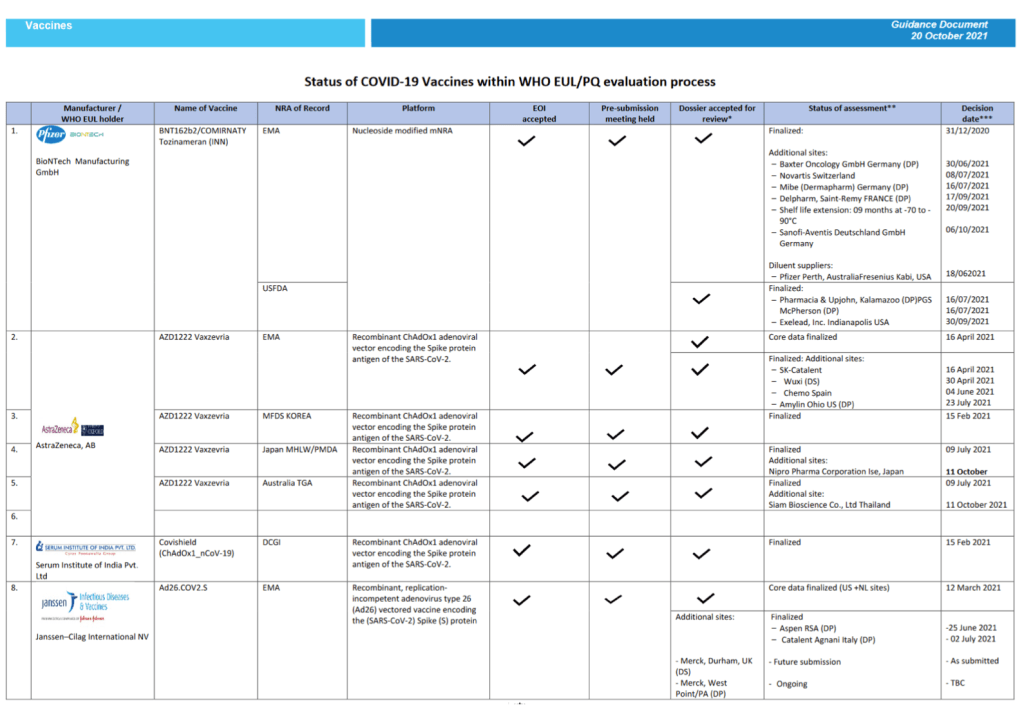
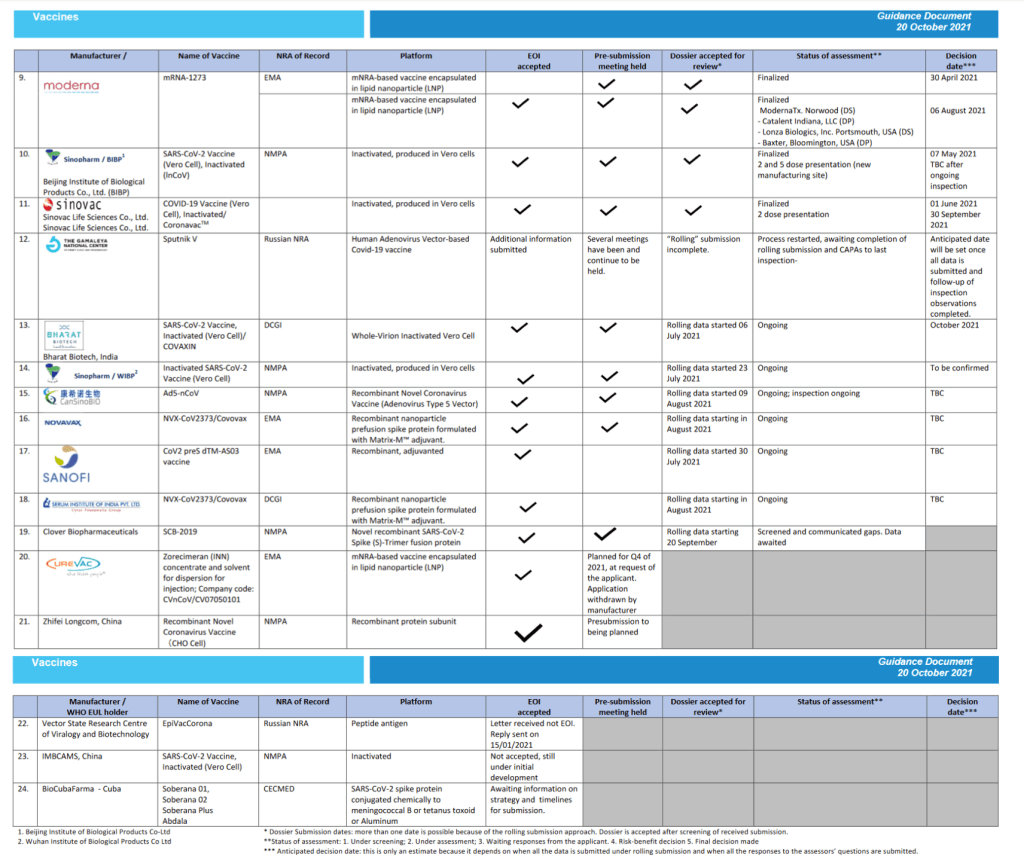
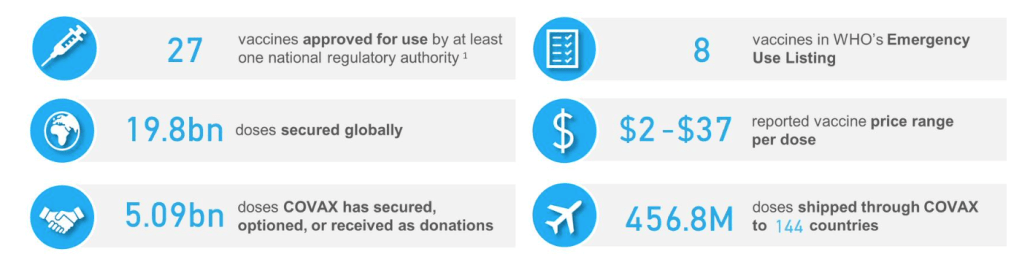
Pre-Print Servers
bioRxiv
https://www.biorxiv.org/
bioRxiv (pronounced “bio-archive”) is a free online archive and distribution service for unpublished preprints in the life sciences. It is operated by Cold Spring Harbor Laboratory, a not-for-profit research and educational institution. By posting preprints on bioRxiv, authors are able to make their findings immediately available to the scientific community and receive feedback on draft manuscripts before they are submitted to journals.
[Accessed 13 Nov 2021]
Potent antibody immunity to SARS-CoV-2 variants elicited by a third dose of inactivated vaccine
Bin Ju, Bing Zhou, Shuo Song, Qing Fan, Xiangyang Ge, Haiyan Wang, Lin Cheng, Huimin Guo, Dan Shu, Lei Liu, Zheng Zhang
bioRxiv 2021.11.10.468037; doi: https://doi.org/10.1101/2021.11.10.468037
Serum from COVID-19 patients early in the pandemic shows limited evidence of cross-neutralization against variants of concern
Marzi, A., Griffin, A., O’Donnell, K. L., Shifflett, K., Lavik, J.-P., Russell, P. M., Zimmerman, M. K., Relich, R. F.
10.1101/2021.11.10.468174 — Posted: 2021-11-12
Gates Open Research
https://gatesopenresearch.org/browse/articles
[Accessed 13 Nov 2021]
[No new digest content identified]
medRxiv
https://www.medrxiv.org/content/about-medrxiv
medRxiv is a free online archive and distribution server for complete but unpublished manuscripts (preprints) in the medical, clinical, and related health sciences. Preprints are preliminary reports of work that have not been certified by peer review. They should not be relied on to guide clinical practice or health-related behavior and should not be reported in news media as established information. medRxiv is for the distribution of preprints – complete but unpublished manuscripts – that describe human health research conducted, analyzed, and interpreted according to scientific principles…
[Accessed 13 Nov 2021]
Selected Content
Protection offered by mRNA-1273 versus BNT162b2 vaccines against SARS-CoV-2 infection and severe COVID-19 in Qatar
Abu-Raddad, L. J., Chemaitelly, H., Ayoub, H. H., Tang, P. J., Hasan, M. R., Coyle, P., YASSINE, H. M., Benslimane, F., Al-Khatib, H. A., Al-Kanaani, Z., Al Kuwari, E., Jeremijenko, A., Kaleeckal, A. H., Latif, A. N., Shaik, R. M., Abdul Rahim, H. F., Nasrallah, G., Al Kuwari, M. G., Butt, A. A., Al Romaihi, H. E., Al-Thani, M. H., Al Khal, A., Bertollini, R.
10.1101/2021.11.12.21266250 — Posted: 2021-11-13
Age and product dependent vaccine effectiveness against SARS-CoV-2 infection and hospitalisation among adults in Norway: a national cohort study, January to September 2021.
Starrfelt, J., Buanes, E. A., Juvet, L. K., Lyngstad, T. M., Ro, G. O. I., Veneti, L., Meijerink, H.
10.1101/2021.11.12.21266222 — Posted: 2021-11-12
Vaccine effectiveness of Pfizer-BioNTech and Oxford-AstraZeneca to prevent severe COVID-19 in Costa Rica by September and October 2021: A nationwide, observational study of hospitalisations prevalence.
Rosero-Bixby, L.
10.1101/2021.11.08.21266087 — Posted: 2021-11-09
Parents intention to vaccinate their 5-11 years old children with the COVID-19: vaccine rates, predictors and the role of incentives
Shmueli, L.
10.1101/2021.11.05.21265900 — Posted: 2021-11-09
Third dose vaccine With BNT162b2 and its response on Long COVID after Breakthrough infections
hoque, a., Rahman, M., Imam, H., Nahar, N., Hasan Chowdhury, F. U.
10.1101/2021.11.08.21266037 — Posted: 2021-11-09
Surveillance of COVID-19 in a Vaccinated Population: A Rapid Literature Review
Egunsola, O., Farkas, B., Flanagan, J., Salmon, C., Mastikhina, L., Clement, F.
10.1101/2021.11.05.21265763 — Posted: 2021-11-08
A need of COVID19 vaccination for children aged <12 years: Comparative evidence from the clinical characteristics in patients during a recent Delta surge (B.1.617.2)
Li, H., Lin, H., Chen, X., Li, H., Li, H., Lin, S., Huang, L., Chen, G., Zheng, G., Wang, S., Hu, X., Huang, H., Tu, H., Li, X., Ji, Y., Zhong, W., Li, Q., Fang, J., Lin, Q., Yu, R., Xie, B.
10.1101/2021.11.05.21265712 — Posted: 2021-11-08
Defining the determinants of under-vaccination in migrant populations in Europe to improve routine and COVID-19 vaccine uptake: a systematic review
Crawshaw, A. F., Farah, Y., Deal, A., Rustage, K., Hayward, S. E., Carter, J., Knights, F., Goldsmith, L. P., Campos-Matos, I., Wurie, F., Majeed, A., Bedford, H., Forster, A. S., Hargreaves, S.
10.1101/2021.11.08.21266058 — Posted: 2021-11-08
Wellcome Open Research [to 13 Nov 2021]
https://wellcomeopenresearch.org/browse/articles
[Accessed 13 Nov 2021]
Wellcome Open Research provides all Wellcome researchers with a place to rapidly publish any results they think are worth sharing. All articles benefit from rapid publication, transparent peer review and editorial guidance on making all source data openly available.
Systematic Review metrics AWAITING PEER REVIEW
The hidden financial burden of healthcare: a systematic literature review of informal payments in Sub-Saharan Africa [version 1; peer review: awaiting peer review]
Evelyn Kabia, Catherine Goodman, Dina Balabanova, Kui Muraya, Sassy Molyneux, Edwine Barasa
Peer Reviewers Invited
Funder: Wellcome
PUBLISHED 08 Nov 2021