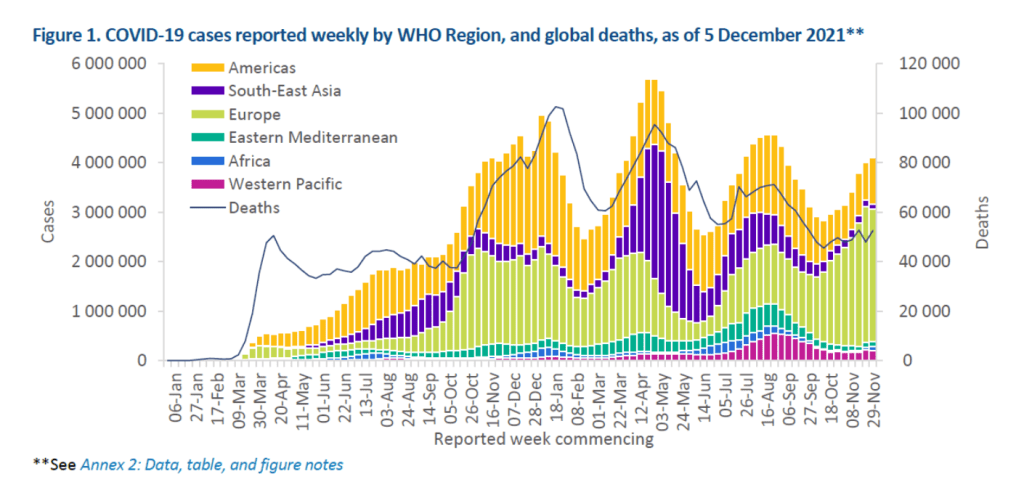
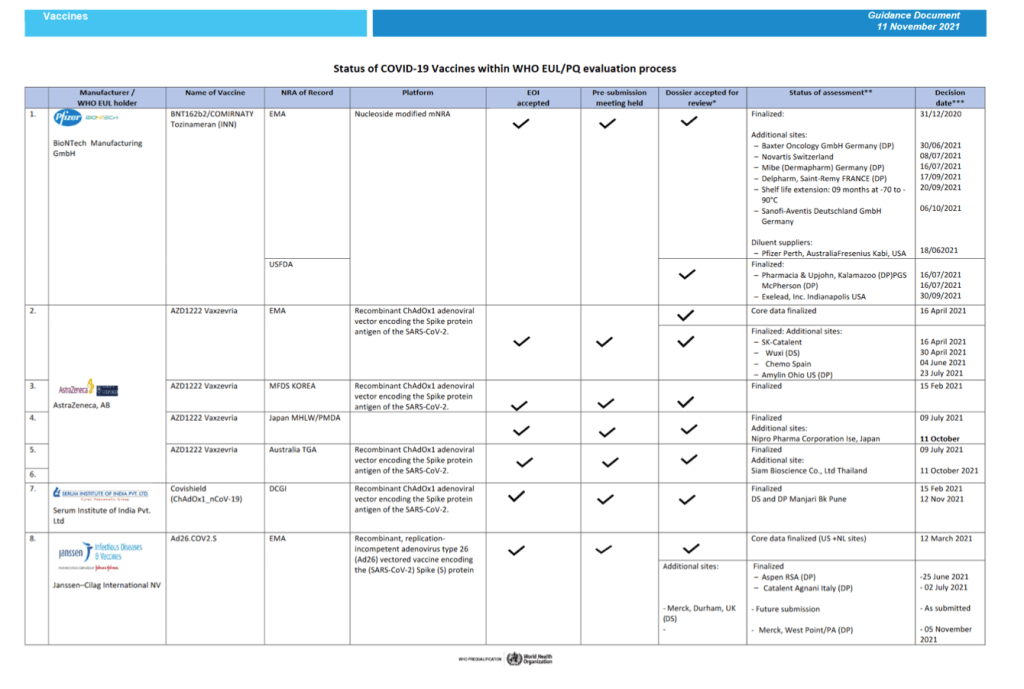
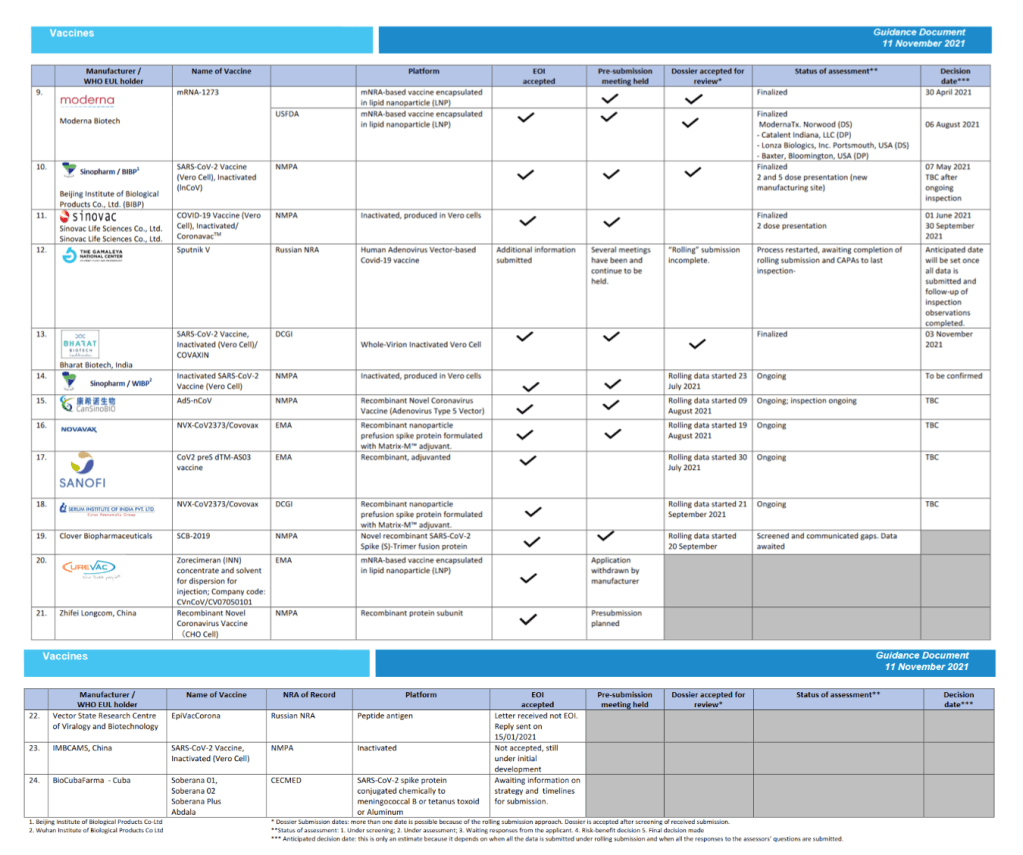
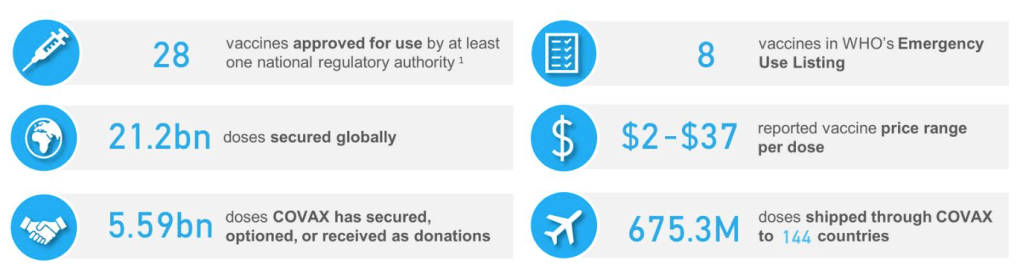
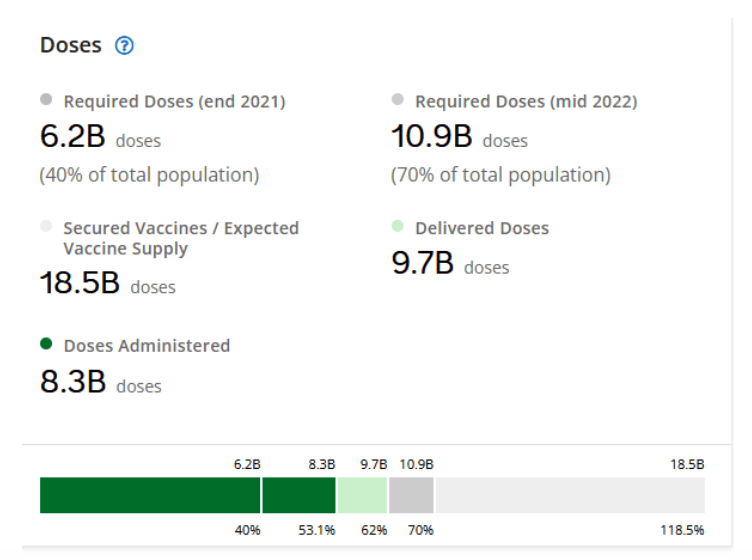
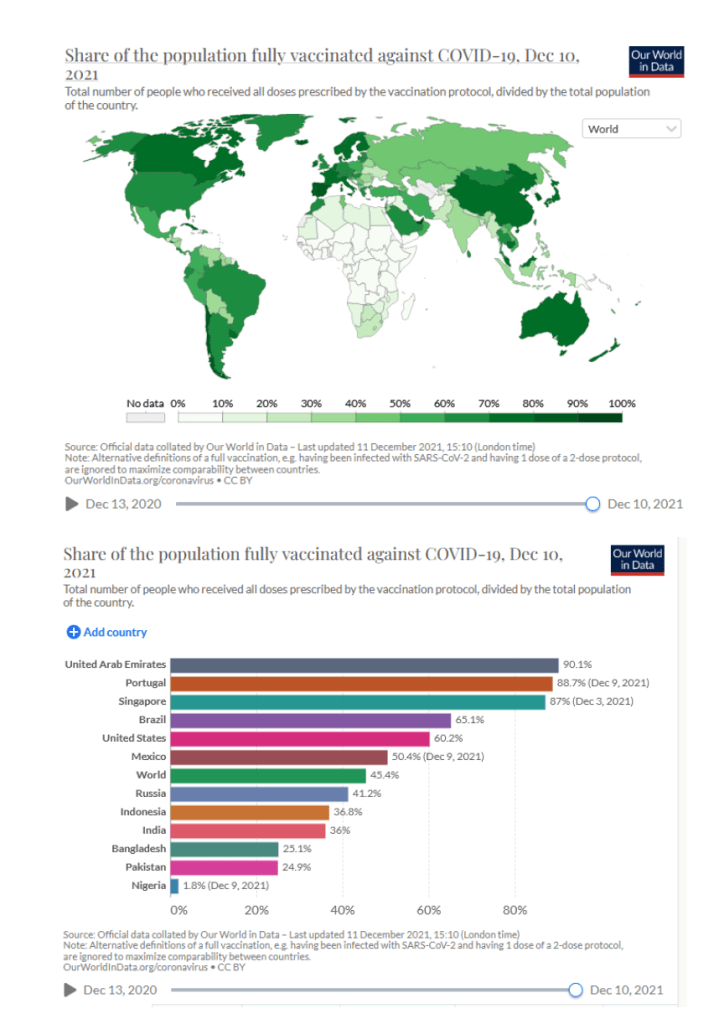
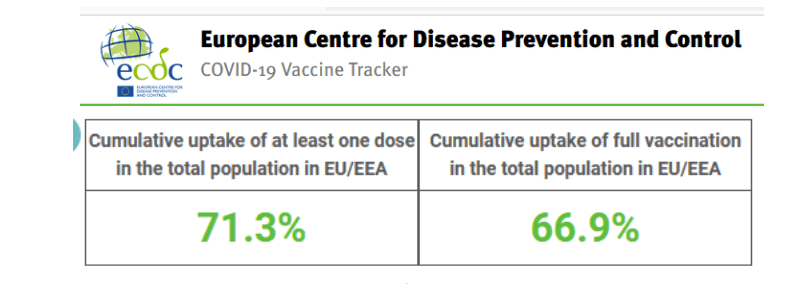
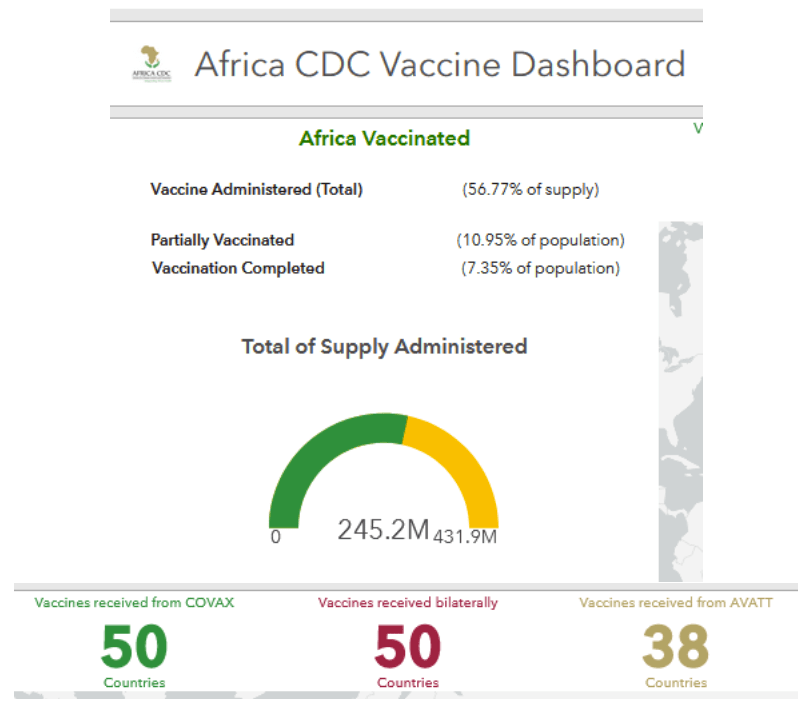
::::::
Organization Announcements
Editor’s Note:
Careful readers will note that the number and range of organizations now monitored in our Announcements section below has grown as the impacts of the pandemic have spread across global economies, supply chains and programmatic activity of multilateral agencies and INGOs.
Paul G. Allen Frontiers Group [to 11 Dec 2021]
https://alleninstitute.org/news-press/
News
No new digest content identified.
BARDA – U.S. Department of HHS [to 11 Dec 2021]
https://www.phe.gov/about/barda/Pages/default.aspx
News
December 08, 2021: FDA Authorizes New Long-Acting Monoclonal Antibodies for Pre-exposure Prevention of COVID-19 in Certain Individuals
December 03, 2021: FDA Expands Authorization of Two Monoclonal Antibodies for Treatment and Post-Exposure Prevention of COVID-19 to Younger Pediatric Patients, Including Newborns
BMGF – Gates Foundation [to 11 Dec 2021]
https://www.gatesfoundation.org/ideas/media-center
Press Releases and Statements
No new digest content identified.
Bill & Melinda Gates Medical Research Institute [to 11 Dec 2021]
https://www.gatesmri.org/
The Bill & Melinda Gates Medical Research Institute is a non-profit biotech organization. Our mission is to develop products to fight malaria, tuberculosis, and diarrheal diseases—three major causes of mortality, poverty, and inequality in developing countries. The world has unprecedented scientific tools at its disposal; now is the time to use them to save the lives of the world’s poorest people
No new digest content identified.
CARB-X [to 11 Dec 2021]
https://carb-x.org/
News
No new digest content identified.
Center for Vaccine Ethics and Policy – GE2P2 Global Foundation [to 11 Dec 2021]
https://centerforvaccineethicsandpolicy.net/
News/Analysis/Statements
:: Past weekly editions and posting of all segments of Vaccines and Global Health: The Week in Review are available here.
:: Informed Consent: A Monthly Review – December 2021 is now posted here
CEPI – Coalition for Epidemic Preparedness Innovations [to 11 Dec 2021]
http://cepi.net/
Latest News
Gritstone bio and CEPI expand vaccine agreement to tackle Omicron variant
06 December, 2021; Emeryville, California and Oslo, Norway—Gritstone bio, Inc. (Nasdaq: GRTS), a clinical-stage biotechnology company developing next generation cancer and infectious disease immunotherapies, and CEPI, the Coalition for Epidemic Preparedness Innovations, today announced the expansion of their agreement in order to support the development of a self-amplifying mRNA (SAM) vaccine designed to tackle the Omicron COVID-19 variant. CEPI will provide up to $5 million in additional funding to conduct a Phase 1 clinical trial of Gritstone’s Omicron vaccine candidate in South Africa, where a CEPI-funded clinical trial of Gritstone’s Beta variant COVID-19 vaccine is due to begin shortly. The SARS-CoV-2 T cell epitopes (TCEs) administered within Gritstone’s SAM COVID-19 vaccines are minimally impacted by mutations found within the Omicron variant, reinforcing the platform’s potential to address both Omicron and future variants of concern…
DARPA – Defense Advanced Research Projects Agency [to 11 Dec 2021
https://www.darpa.mil/news
News
12/8/2021
DARPA Successfully Transitions Synthetic Biomanufacturing Technologies to Support National Security Objectives
Launched in 2010, DARPA’s Living Foundries program aimed to enable adaptable, scalable, and on-demand production of critical, high-value molecules by programming the fundamental metabolic processes of biological systems to generate a vast number of complex molecules. These molecules were often prohibitively expensive, unable to be domestically sourced, and/or impossible to manufacture using traditional synthetic chemistry approaches. As a proof of concept, DARPA intended to produce 1,000 molecules and material precursors spanning a wide range of defense-relevant applications including industrial chemicals, fuels, coatings, and adhesives.
12/6/2021
Leveraging AI to Accelerate Development of Scientific Models
In August 2018, DARPA released its first AI Exploration (AIE) opportunity called Automating Scientific Knowledge Extraction (ASKE). Unlike DARPA’s typical four-year programs, AIEs are designed to be fast-tracked (~18 months in duration) research efforts that help determine the feasibility of an AI concept. The goal of the ASKE project was to develop AI technologies capable of automating some of the manual processes of scientific knowledge discovery, curation, and application. It identified how and where AI could accelerate the process of scientific modeling, and ultimately improve researchers’ ability to conduct rigorous and timely experimentation and validation.
Duke Global Health Innovation Center [to 11 Dec 2021]
https://dukeghic.org/
Our Blog
No new digest content identified.
EDCTP [to 11 Dec 2021]
http://www.edctp.org/
The European & Developing Countries Clinical Trials Partnership (EDCTP) aims to accelerate the development of new or improved drugs, vaccines, microbicides and diagnostics against HIV/AIDS, tuberculosis and malaria as well as other poverty-related and neglected infectious diseases in sub-Saharan Africa, with a focus on phase II and III clinical trials
News
No new digest content identified.
Emory Vaccine Center [to 11 Dec 2021]
http://www.vaccines.emory.edu/
Vaccine Center News
No new digest content identified.
European Vaccine Initiative [to 11 Dec 2021]
http://www.euvaccine.eu/
Latest News News, Events
No new digest content identified.
Fondation Merieux [to 11 Dec 2021]
http://www.fondation-merieux.org/
News, Events
No new digest content identified.
Gavi [to 11 Dec 2021]
https://www.gavi.org/
News Releases
10 December 2021
Gavi and Moderna reach agreement for additional supply to COVAX
:: Agreement will make up to an additional 150 million doses of the Moderna COVID-19 vaccine available to COVAX Facility participants in Q2 and Q3 2022, at the lowest tiered price
:: Gavi and Moderna have also agreed to advance availability of 20 million doses – originally scheduled to be available in Q1 2022 – to Q4 2021
GHIT Fund [to 11 Dec 2021]
https://www.ghitfund.org/newsroom/press
GHIT was set up in 2012 with the aim of developing new tools to tackle infectious diseases that
No new digest content identified.
Global Fund [to 11 Dec 2021]
https://www.theglobalfund.org/en/news/
News & Stories
News
WHO and Global Fund Warn Inequalities Block Progress Towards Ending AIDS, TB and Malaria
09 December 2021
[See Perspectives above for detail]
Global Research Collaboration for Infectious Disease Preparedness [GloPID-R] [to 11 Dec 2021]
https://www.glopid-r.org/news/
News
No new digest content identified.
Hilleman Laboratories [to 11 Dec 2021]
http://www.hillemanlabs.org/
News & Insights
Press Release
Hilleman Laboratories establishes pilot manufacturing facility for vaccine development in Singapore with support from Singapore Economic Development Board
New facility is part of first-of-its-kind vaccine development and manufacturing hub in Singapore
Singapore, 6 December 2021: Hilleman Laboratories, an organisation with a mission to develop affordable vaccines and biologics against infectious diseases, today announced a first-of-its-kind vaccine and biologics development and manufacturing hub in Singapore. Established with the support of the Singapore Economic Development Board (EDB), the hub comprises a 30,000 square foot current Good Manufacturing Practices (cGMP) facility at 138 Depot Road and a state-of-the art research and development (R&D) facility at Biopolis which began operations in April 2021. Hilleman Laboratories is investing close to SGD 80 million (USD 58 million) in operations, infrastructure and capacity building over the next five years…
HHMI – Howard Hughes Medical Institute [to 11 Dec 2021]
https://www.hhmi.org/news
Press Room
No new digest content identified
Human Vaccines Project [to 11 Dec 2021]
http://www.humanvaccinesproject.org/
News
HVP COVID Report
Dec 09, 2021
Wayne Koff: Harnessing the Power of AI to Understand Human Immunity
IAVI [to 11 Dec 2021]
https://www.iavi.org/newsroom
Latest News
No new digest content identified.
International Coalition of Medicines Regulatory Authorities [ICMRA]
http://www.icmra.info/drupal/en/news
Selected Statements, Press Releases, Research
No new digest content identified.
ICRC [to 11 Dec 2021]
https://www.icrc.org/en/whats-new
Selected News Releases, Statements, Reports
South Sudan: Looting of a health care unit during armed violence in Unity State
People wounded during the clashes have had to walk up to 30km across heavily flooded terrain to access health care following the looting of Padeah Primary Health Unit.
09-12-2021 | Statement
Dakar Forum on Peace and Security: “There can be no security without human security”
The following comments were made by Peter Maurer, president of the International Committee of the Red Cross, as part of a panel discussion on challenges to stability and emergence in Africa in a post-COVID-19 world, at the Dakar International Forum o
06-12-2021 | Statement
IFFIm
http://www.iffim.org/
Press Releases/Announcements
No new digest content identified.
IFRC [to 11 Dec 2021]
http://media.ifrc.org/ifrc/news/press-releases/
Selected Press Releases, Announcements
09/12/2021
Ethiopia: IFRC steps up emergency response amid “ove…
06/12/2021
IFRC President Francesco Rocca meets with Russian Pr…
Moscow/Geneva, 6 December 2021 – The President of the International Federation of Red Cross and Red Crescent Societies (IFRC) Francesco Rocca, met in the late afternoon of the 5th of December with the President of the Russian Federation, Vladimir Putin.
During the meeting, President Rocca discussed humanitarian priorities and challenges, such as; the COVID-19 pandemic, the equitable distribution of vaccines, the migration crisis at the border between Belarus and Poland, how to strengthen support to the Russian Red Cross, and the critical role of local actors to respond to emergencies…
06/12/2021
Indonesia: Rescue and relief critical after Mount Se…
Institut Pasteur [to 11 Dec 2021]
https://www.pasteur.fr/en/press-area
Press Documents
Press Info
01.12.2021
Virus related to SARS-CoV-2 found in bats in Cambodia
What are the origins of SARS-CoV-2? This is the world’s most pressing scientific puzzle. Its pieces scattered throughout the world are currently being sought out by scientists. Their goal: to track down the culprit for the largest health crisis to hit the globe in the past century. Scientists at the Institut Pasteur in Paris and the Institut Pasteur du Cambodge have identified a coronavirus in bats in Cambodia whose genome is 92.6% related to SARS-CoV-2. These findings were published in Nature Communications on November 9, 2021.
IOM / International Organization for Migration [to 11 Dec 2021]
http://www.iom.int/press-room/press-releases
News – Selected
News
10 Dec 2021
Rising Migrant Deaths Top 4,400 This Year: IOM Records More Than 45,000 Since 2014
ISC / International Science Council [to 11 Dec 2021]
https://council.science/current/
ISC is a non-governmental organization with a unique global membership that brings together 40 international scientific Unions and Associations and over 140 national and regional scientific organizations including Academies and Research Councils.
News
News Press releases
Researchers combat new threats to key human rights
10.12.2021
Scientists call for global science systems that protect the human right to science in a new paper published today by the International Science Council (ISC).
Report: https://stories.council.science/science-freedom-responsibility/
Press releases
Global Commission on Science Missions for Sustainability Launched
09.12.2021
Political leaders, scientists, and influential personalities issue emergency warning on sustainability inaction, establish a Global Commission to mobilize a $100 million a year global fund for Sustainability Science Missions
Commission members include former NZ PM Helen Clark, former UNESCO Director General Irina Bokova, film producer James Cameron and philanthropist Julie Wrigley
9 December 2021 Paris, France: The International Science Council (ISC) is a non-governmental organization with a unique global membership bringing together over 200 international scientific unions and associations, as well as national and regional scientific organizations, academies, and research councils. Today in Paris, its new President, Peter Gluckman, former Chief Science Adviser to the government of New Zealand, announced the ‘Global Commission on Science Missions for Sustainability’ with the sole purpose of pressing the reset button to accelerate the progress towards the Sustainable Development Goals (SDGs).
IVAC [to 11 Dec 2021]
https://www.jhsph.edu/research/centers-and-institutes/ivac/index.html
Updates; Events
No new digest content identified.
IVI [to 11 Dec 2021]
http://www.ivi.int/
IVI News & Announcements
IVI, ADB SEADS, IPK, ICARS, and the Danish Embassy in Korea launch webinar on combatting antimicrobial resistance
December 7, 2021 — The International Vaccine Institute (IVI), Asian Development Bank Southeast Asia Development Solutions (ADB SEADS), Institut Pasteur Korea (IPK), the International Centre for Antimicrobial Resistance Solutions (ICARS), and the Embassy of Denmark in Korea held a joint webinar today to bring awareness to antimicrobial resistance (AMR) and discuss sustainable solutions to the global public health threat…
Johns Hopkins Center for Health Security [to 11 Dec 2021]
https://www.centerforhealthsecurity.org/news/center-news/
Center News
2021 Global Health Security Index Finds All Countries Remain Dangerously Unprepared for Future Epidemic and Pandemic Threats
December 8, 2021
[See Perspectives above for detail]
MSF/Médecins Sans Frontières [to 11 Dec 2021]
http://www.msf.org/
Latest [Selected Announcements]
DRC Ebola outbreaks
DRC’s thiteenth Ebola outbreak
Crisis Update 9 Dec 2021
Following a series of community deaths in North Kivu province, Democratic Republic of Congo (DRC), the DRC Ministry of Health declared an outbreak of Ebola on 8 October. The outbreak is concentrated in Beni health zone. This is the thirteenth epidemic in DRC, the third in North Kivu province and the second in Beni health zone.
Coronavirus COVID-19 pandemic
Responding to COVID-19: Global Accountability Report 5 – May to September 2021
Report 8 Dec 2021
Kenya
Urgent solutions needed for refugees as camps set to close
Press Release 6 Dec 2021
Kenya
In search of dignity: Refugees in Kenya face a reckoning
Report 6 Dec 2021
National Academy of Medicine – USA [to 11 Dec 2021]
https://nam.edu/programs/
Selected News/Programs/Events
No new digest content identified.
National Academy of Sciences – USA [to 11 Dec 2021]
http://www.nasonline.org/news-and-multimedia/
News
No new digest content identified.
National Vaccine Program Office – U.S. HHS [to 11 Dec 2021]
https://www.hhs.gov/vaccines/about/index.html
Upcoming Meetings/Latest Updates
No new digest content identified.
NIH [to 11 Dec 2021]
http://www.nih.gov/news-events/news-releases
News Releases
Experimental mRNA HIV vaccine safe, shows promise in animals
December 9, 2021 — NIH scientists developed vaccine platform.
An experimental HIV vaccine based on mRNA—the same platform technology used in two highly effective COVID-19 vaccines—shows promise in mice and non-human primates, according to scientists at the National Institute of Allergy and Infectious Diseases (NIAID), part of the National Institutes of Health. Their results, published in Nature Medicine, show that the novel vaccine was safe and prompted desired antibody and cellular immune responses against an HIV-like virus…
OECD [to 11 Dec 2021]
http://www.oecd.org/newsroom/publicationsdocuments/bydate/
Newsroom
Portugal: use post-COVID-19 recovery plan to bolster growth and public finances, says OECD
Portugal’s economy is recovering from the COVID-19 crisis, thanks to swift and effective policy action and a successful vaccine rollout. As the recovery progresses, it is important to pursue investment and structural reforms that will raise living standards, strengthen public finances and put growth on a strong, sustainable and resilient path, according to a new OECD report.
10-December-2021
Pensions protected during COVID-19 pandemic but ageing challenges persist, says OECD
The COVID-19 pandemic has taken a heavy toll among elderly people although retirees have seen their pension payments well protected across OECD countries. Future pension entitlements have also been well protected thanks to the exceptional policy response to the crisis, according to a new OECD report.
8-December-2021
PATH [to 11 Dec 2021]
https://www.path.org/media-center/
Press Releases
No new digest content identified.
Sabin Vaccine Institute [to 11 Dec 2021]
http://www.sabin.org/updates/pressreleases
Statements and Press Releases
No new digest content identified.
UNAIDS [to 11 Dec 2021]
http://www.unaids.org/en
Selected Press Releases/Reports/Statements
10 December 2021
Co-creating a new global initiative to end AIDS among children, adolescents and their mothers
10 December 2021
The need for wider implementation of people-centred differentiated service delivery for HIV testing and treatment in Africa
9 December 2021
High-level continental seminar on the right to health and social protection in Africa
9 December 2021
Social protection systems addressing inequalities
8 December 2021
Empowering sex workers through social enterprises
8 December 2021
We need your thoughts and ideas on how to end AIDS among children, adolescents and mothers—new global online survey launched
8 December 2021
Leaders from Eastern and Southern Africa recommit to the education, health and well-being of adolescents and young people
7 December 2021
The importance of comprehensive sexuality education for Africa’s young people
6 December 2021
Commemorating World AIDS Day in the Central African Republic
UNHCR Office of the United Nations High Commissioner for Refugees [to 11 Dec 2021]
http://www.unhcr.org/en-us/media-centre.htmlS
Selected News Releases, Announcements
No new digest content identified.
UNICEF [to 11 Dec 2021]
https://www.unicef.org/media/press-releases
Press Releases, News Notes, Statements [Selected]
Press release
12/10/2021
UNICEF welcomes announcement of next Executive Director
NEW YORK, 10 December 2021 – UNICEF welcomes United Nations Secretary-General António Guterres’ announcement today that Catherine M. Russell will succeed Henrietta H. Fore as UNICEF Executive Director.
Ms. Russell brings to the role decades of experience in developing innovative policy that empowers underserved communities around the world; delivering high-impact programmes that protect women and girls, including in humanitarian crises; building, elevating, and managing diverse workforces; and mobilizing resources and political support for a broad range of initiatives.
She currently serves as Assistant to the President and Director of the White House Office of Presidential Personnel. From 2013 to 2017, she served as Ambassador-at-Large for Global Women’s Issues at the U.S. Department of State. In that post, she integrated women’s issues across all elements of U.S. foreign policy, represented the United States in more than 45 countries, and worked with foreign governments, multilateral organizations, and civil society. She was the principal architect of the ground-breaking “U.S. Global Strategy to Empower Adolescent Girls”…
Press release
12/08/2021
COVID-19 ‘biggest global crisis for children in our 75-year history’ – UNICEF
On its 75th anniversary, UNICEF warns that COVID-19 is rolling back virtually every measure of progress for children, including a staggering 100 million more children plunged into poverty
Press release
12/08/2021
More than US$27 billion committed to tackle global malnutrition and hunger crisis at the Tokyo Nutrition for Growth Summit
45 low- and middle-income countries led with strong policy and financial commitments
Press release
12/07/2021
UNICEF launches US$9.4 billion emergency funding appeal for children affected by conflict, the climate crisis and COVID-19
Funds will support essential programs for over 177 million children in need across 145 countries and territories through 2022
News note
12/07/2021
UNICEF signs supply agreement for Clover COVID-19 vaccine
NEW YORK/COPENHAGEN, 7 December 2021 – UNICEF and Clover Biopharmaceuticals Ltd. have signed a long-term agreement for the supply of the Clover COVID-19 vaccine on behalf of the COVAX Facility.
Through the supply agreement, UNICEF will have access to up to 414 million doses of Clover’s COVID-19 vaccine until the end of 2022. These doses were committed to the COVAX Facility through an Advance Purchase Agreement (APA) signed between Gavi, the Vaccine Alliance and Clover Biopharmaceuticals on 30 June 2021. The vaccine will be supplied to participating countries and territories in the COVAX Facility’s Advance Market Commitment (AMC), as well as self-financing participants.
This agreement further expands UNICEF’s COVID-19 vaccine supplier base. UNICEF has already signed supply agreements with vaccine manufacturers including the Serum Institute of India, Pfizer, AstraZeneca, Human Vaccine, Moderna, Janssen Pharmaceutica NV, Sinopharm and Sinovac. The addition of Clover’s vaccine to the portfolio provides further diversification by providing access to the first protein subunit vaccine.
Procurement by UNICEF under this agreement is conditional on the product achieving an Emergency Use Listing (EUL) from WHO, to confirm the quality, safety and efficacy of the vaccine…
Statement
12/06/2021
UNICEF and WHO, in partnership with Gavi, ask Ted Chaiban to serve as Global Lead Coordinator for COVID Vaccine Country Readiness and Delivery
NEW YORK, 6 December 2021 – “I am pleased to announce that UNICEF and WHO, in partnership with Gavi, have asked Ted Chaiban, currently UNICEF Regional Director Middle East and North Africa, to serve as Global Lead Coordinator for COVID Vaccine Country Readiness and Delivery.
“This decision is an expression of our joint commitment to reinvigorate efforts to turn vaccines into vaccinations in low- and middle-income countries.
“With vastly improved supply, the world no longer has a global vaccine supply problem; it has a vaccine equity and delivery problem. More than 80 per cent of the world’s vaccines have gone to G20 countries while millions of people, including healthcare workers, in low-income countries are still unprotected.
“As vaccine doses become increasingly available in the coming weeks, it is critical that countries are prepared, resourced and able to roll them out.
“With support from COVAX, the African Union, AVAT and other partners, governments in low-income countries have made important progress in getting ready to receive and administer COVID-19 vaccines at a scale sufficient to meet global objectives. However, they still face significant challenges in scaling up capacity….
Press release
12/06/2021
Learning losses from COVID-19 could cost this generation of students close to $17 trillion in lifetime earnings
World Bank-UNESCO-UNICEF report lays out the magnitude of the education crisis
Unitaid [to 11 Dec 2021]
https://unitaid.org/
Featured News
09 December 2021
Landscape report launched on novel technologies for the prevention of HIV, STIs, and unwanted pregnancy
Geneva – Emerging technologies that can prevent HIV, other sexually transmitted infections and unwanted pregnancies through a single administration method have the potential to respond better to user preferences, simplify service delivery, alleviate stigma, and reduce health risks. Currently, over two dozen of these tools, called multipurpose prevention technologies, are in development, demonstrating the opportunity to accelerate health impact and help achieve global health targets, according to a new landscape report jointly launched today by Unitaid, the Children’s Investment Fund Foundation, and the Initiative for Multipurpose Prevention Technologies…
Vaccine Equity Cooperative [nee Initiative] [to 11 Dec 2021]
https://vaccineequitycooperative.org/news/
News
No new digest content identified.
Vaccination Acceptance & Demand Initiative [Sabin) [to 11 Dec 2021]
https://www.vaccineacceptance.org/
Announcements
Focusing on Marginalized Communities
Get to Know Five of Our 2021 Social and Behavioral Research Grant Partners
December 9, 2021
By: Abigail Quinn, BA, Deeva Agravat, MSc, Kate Hopkins, PhD, MPH
The Sabin Vaccine Institute’s Vaccine Acceptance & Demand initiative is proud to provide funding to 10 grant partners awarded through the 2021 Social and Behavioral Research Grants Program. This blog is the second in a series examining the selected research projects based on one of three themes: vaccine equity, marginalized communities and social media/messaging…
Vaccine Confidence Project [to 11 Dec 2021]
http://www.vaccineconfidence.org/
News, Research and Reports
No new digest content identified.
Vaccine Education Center – Children’s Hospital of Philadelphia [to 11 Dec 2021]
http://www.chop.edu/centers-programs/vaccine-education-center
News
No new digest content identified.
Wellcome Trust [to 11 Dec 2021]
https://wellcome.ac.uk/news
News. Opinion, Reports
News
People worldwide want their governments to value science
During a global pandemic, it’s vital that governments endorse and implement scientific recommendations. We asked people around the world whether they felt their government valued scientific advice throughout the coronavirus pandemic.
8 December 2021
The Wistar Institute [to 11 Dec 2021]
https://www.wistar.org/news/press-releases
Press Releases
No new digest content identified.
WFPHA: World Federation of Public Health Associations [to 11 Dec 2021]
https://www.wfpha.org/
Latest News – Blog
Human Rights Day 2021
Dec 10, 2021
…The International Federation of Social Workers and World Federation of Public Health Association jointly advance a statement to continue to advocate with the United Nations, governments, companies and civil societies for the promotion of equity and respect for human rights, starting with the equitable distribution of vaccines and guaranteeing the universal right to sustainable development…
Statement link: https://drive.infomaniak.com/app/share/141741/8c4e56c4-6620-4c77-8095-e967707d8c8a/preview/pdf/39843
World Bank [to 11 Dec 2021]
http://www.worldbank.org/en/news/all
Selected News, Announcements
Ukraine to Expand COVID-19 Vaccination, with Additional World Bank Financing
WASHINGTON, December 10, 2021 – The World Bank’s Board of Executive Directors approved today $150 million in Additional Financing for the Ukraine Emergency COVID-19 Response and Vaccination Project…
Date: December 10, 2021 Type: Press Release
Learning Losses from COVID-19 Could Cost this Generation of Students Close to $17 Trillion in Lifetime Earnings
World Bank-UNESCO-UNICEF report lays out the magnitude of the education crisis WASHINGTON, DC, Dec. 6, 2021—This generation of students now risks losing $17 trillion in lifetime earnings in present…
Date: December 06, 2021 Type: Press Release
The State of the Global Education Crisis: A Path to Recovery
The global disruption to education caused by the COVD-19 pandemic is without parallel and the effects on learning are severe. The crisis brought education systems across the world to a halt, with school…
Date: December 03, 2021 Type: Publication
World Organisation for Animal Health (OIE) [to 11 Dec 2021]
https://www.oie.int/en/media/news/
Press Releases, Statements
OIE Statement on monitoring white-tailed deer for SARS-CoV-2
Statements
3 December 2021
Recent scientific research has shown a high prevalence of SARS-CoV-2 infection within white-tailed deer populations in North America. This is the first time that the virus has been detected at population levels in wildlife. This discovery requires further research to determine if white-tailed deer could become a reservoir of SARS-CoV-2 and to assess other animal or public health implications. As they do not show clinical signs of infection, white-tailed deer should be monitored for the possibility of becoming a silent reservoir.
While there is currently no evidence of transmission of SARS-CoV-2 from white tailed-deer to humans, there appears to have been multiple introductions of the virus into white-tailed deer populations by humans. We encourage countries to raise awareness with both hunters, and those living or working with wildlife, to avoid unnecessary interactions with wildlife and to avoid leaving any human waste or objects in forested areas that may be ingested or touched by deer and other wildlife…
WTO – World Trade Organisation [to 11 Dec 2021]
http://www.wto.org/english/news_e/news_e.htm
WTO News and Events
Monitoring report shows continued but slow rollback of COVID-19 related trade restrictions
9 December 2021
WTO members showed restraint in the imposition of new trade-restrictive measures related to COVID-19 and continued to roll back restrictions adopted earlier in the pandemic, according to the Director-General’s annual overview report on trade-related developments. Despite a slowdown in new trade restrictions unrelated to the pandemic, the stockpile of unrepealed restrictive measures that has accumulated since the monitoring exercise started in 2009 now affects traded merchandise worth an estimated USD 1.5 trillion, or nearly 9% of world imports.
::::::
ARM [Alliance for Regenerative Medicine] [to 11 Dec 2021]
https://alliancerm.org/press-releases/
Selected Press Releases
No new digest content identified.
BIO [to 11 Dec 2021]
https://www.bio.org/press-releases
Press Releases, Letters, Testimony, Comments [Selected]
No new digest content identified.
DCVMN – Developing Country Vaccine Manufacturers Network [to 11 Dec 2021]
http://www.dcvmn.org/
News; Upcoming events
Dr. Suresh Jadhav (1949-2021)
Pune, 07 December 2021 – Today was a sad day for all in the vaccine industry with the passing away of Dr. Suresh Jadhav: a humble, friendly yet very knowledgeable and highly respected person. His warm smile and kind words are still on our minds.
We convey our heartiest and sincere condolences to his family and close friends on his loss and pay tribute to his exemplary lifetime and dedication to vaccines and immunization.
He was a great vaccinologist and one of the persons with a pivotal role, for over 40 years, in building the Serum Institute of India global outreach, in getting WHO pre-qualification of several products.
Dr. Jadhav was also instrumental in leading the Developing Countries Vaccine Manufacturers Network (DCVMN) into its current status and we owe him a huge debt of gratitude.
We shall continue our work in respect of his life, legacy and memory.
DCVMN members
ICBA – International Council of Biotechnology Associations [to 11 Dec 2021]
https://internationalbiotech.org/news/
News
No new digest content identified.
IFPMA [to 11 Dec 2021]
http://www.ifpma.org/resources/news-releases/
Selected Press Releases, Statements, Publications
Industry Position Paper: Optimising Post-Approval Change Management to Facilitate Continuous Supply of Medicines and Vaccines
10 December 2021
Industry Position
:: Industry acknowledges its commitment to continue improving its strategic and predictive planning and proactive communication of changes to help facilitate global supply.
:: In addition, Industry believes that global regulatory convergence of post-approval changes to Marketing Authorisations (MAs) using science and risk-based approaches will enable a more efficient management of quality and supply improvements and will facilitate patients’ access to innovative medicines and vaccines.
:: National Regulatory Authorities (NRAs) should: establish national or regional guidelines in line with
international standards (with regard to a risk based classification of changes and standardization of
requirements) [1, 2]; have clear procedural guidance including timelines; and implement reliance
pathways to accelerate the approval of changes
[1] – WHO Annex 3—Guidelines on procedures and data requirements for changes to approved
biotherapeutic products WHO Technical Report Series N° 1011, 2018
[2] – WHO Annex 4: Guidelines on procedures and data requirements for changes to approved vaccines WHO Technical Report Series N° 993, 2015
International Generic and Biosimilar Medicines Association [IGBA]
https://www.igbamedicines.org/
News
No new digest content identified.
International Alliance of Patients’ Organizations – IAPO [to 11 Dec 2021]
https://www.iapo.org.uk/news/topic/6
Press and media [Selected]
No new digest content identified.
PhRMA [to 11 Dec 2021]
http://www.phrma.org/
Latest News [Selected]
No new digest content identified.