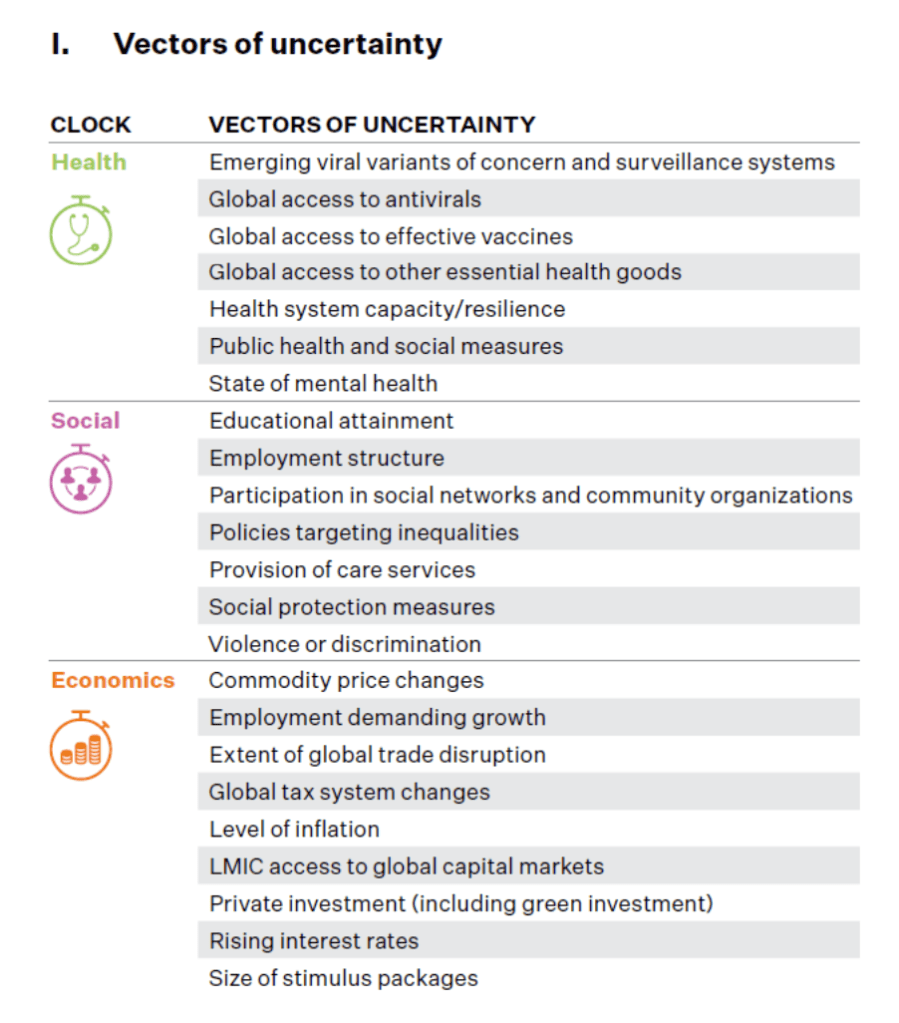
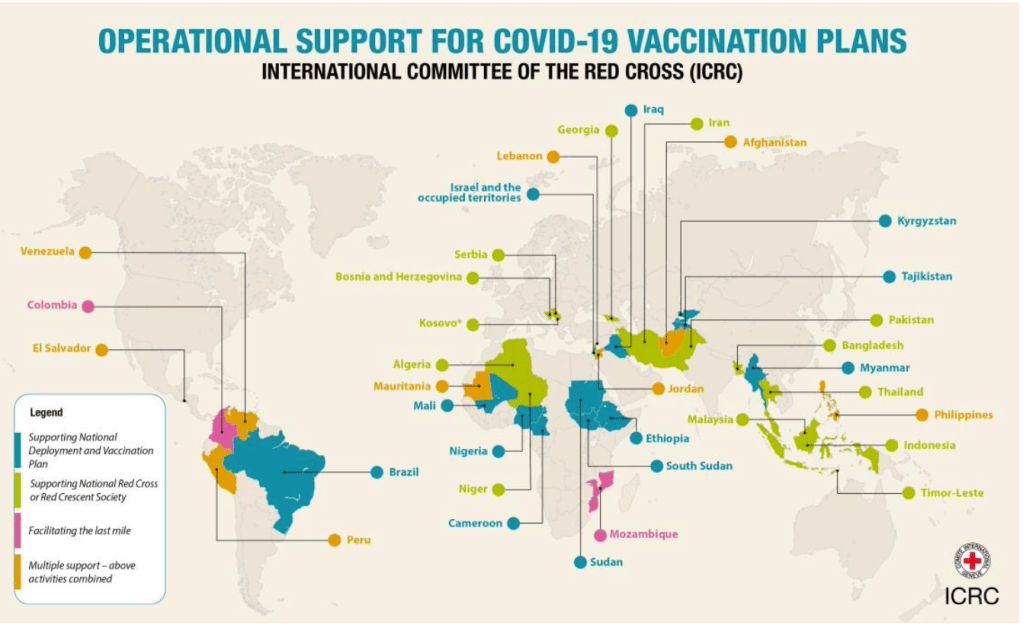
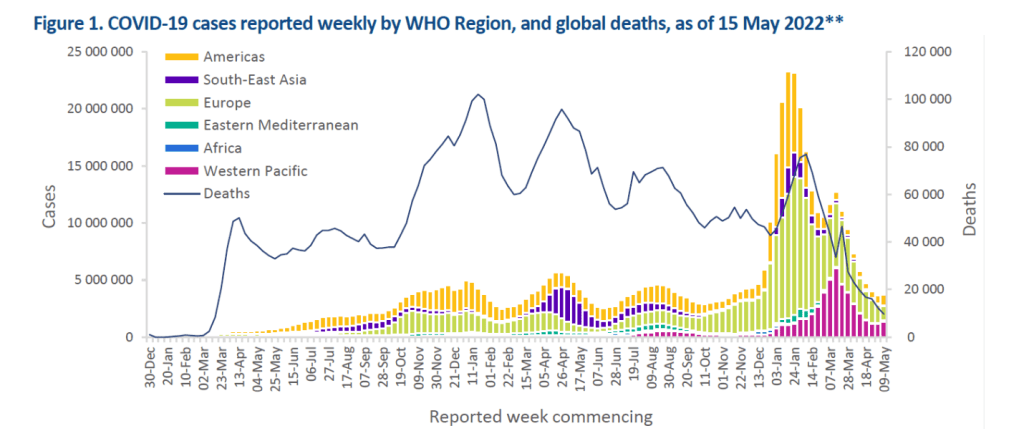
COVID Perspectives
COVAX calls for urgent action to close vaccine equity gap
This is a joint statement from the COVAX co-leads CEPI, Gavi, WHO, and UNICEF.
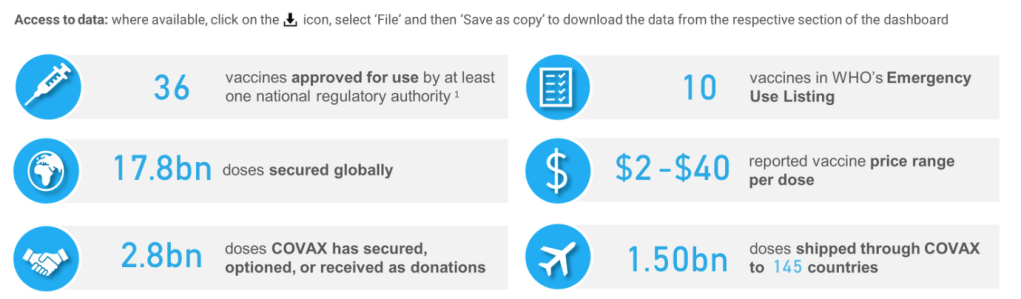
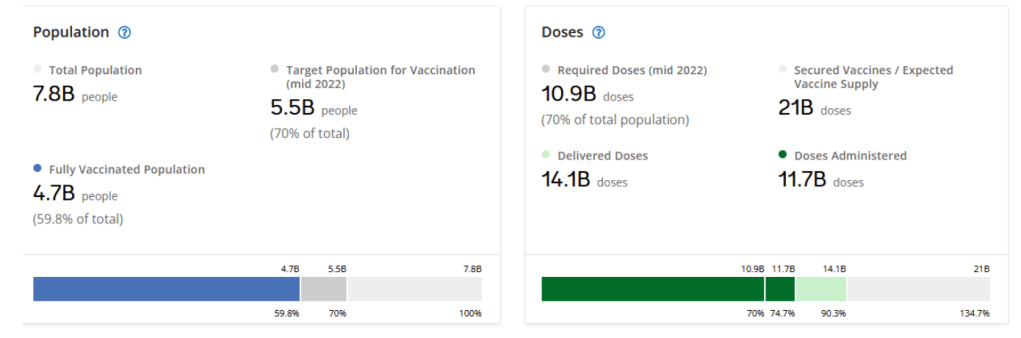
– COVAX has access to enough COVID-19 vaccines to help protect 70% of the population in 91 lower income countries
– Demand and uptake are low, with low-income countries remaining furthest behind
– To close the global vaccine equity gap, COVAX calls on countries to set ambitious targets for implementation and on all partners to ensure countries have the resources needed to accelerate and expand national strategies
Geneva/New York/Oslo, 20 May 2022 – Nearly 18 months after the first administration of a COVID-19 vaccine, incredible progress has been made – with lower-income countries administering billions of COVID-19 vaccines in a historic global rollout that is unprecedented in terms of speed, scale and demographics reached. Yet despite this progress, and the easing of global supply constraints, inequities between lower and higher income countries are continuing to cost lives and are prolonging the pandemic by increasing the threat posed by the emergence of new, potentially more dangerous variants of the virus.
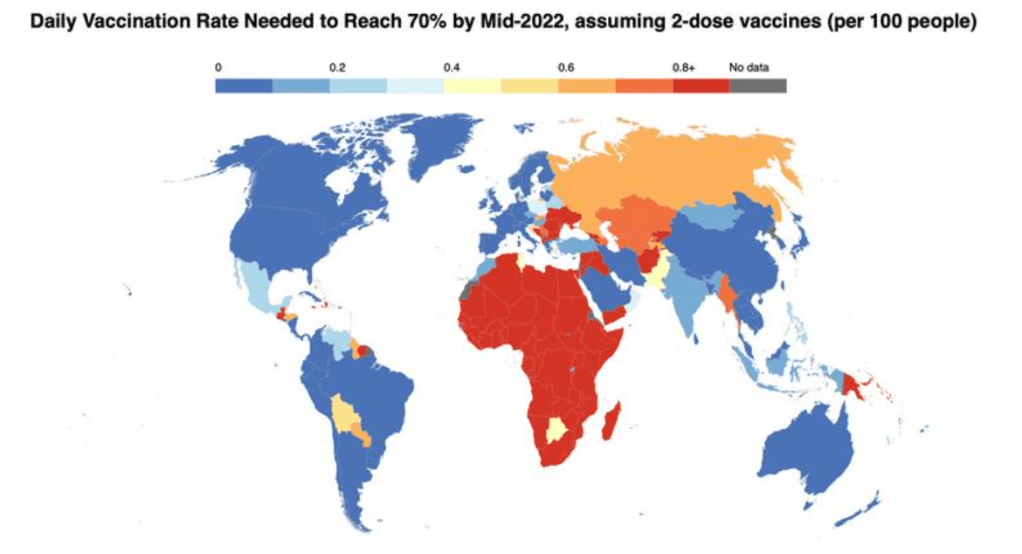
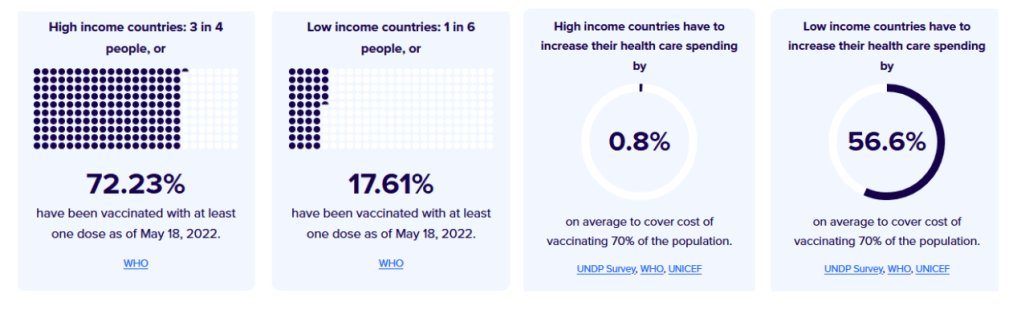
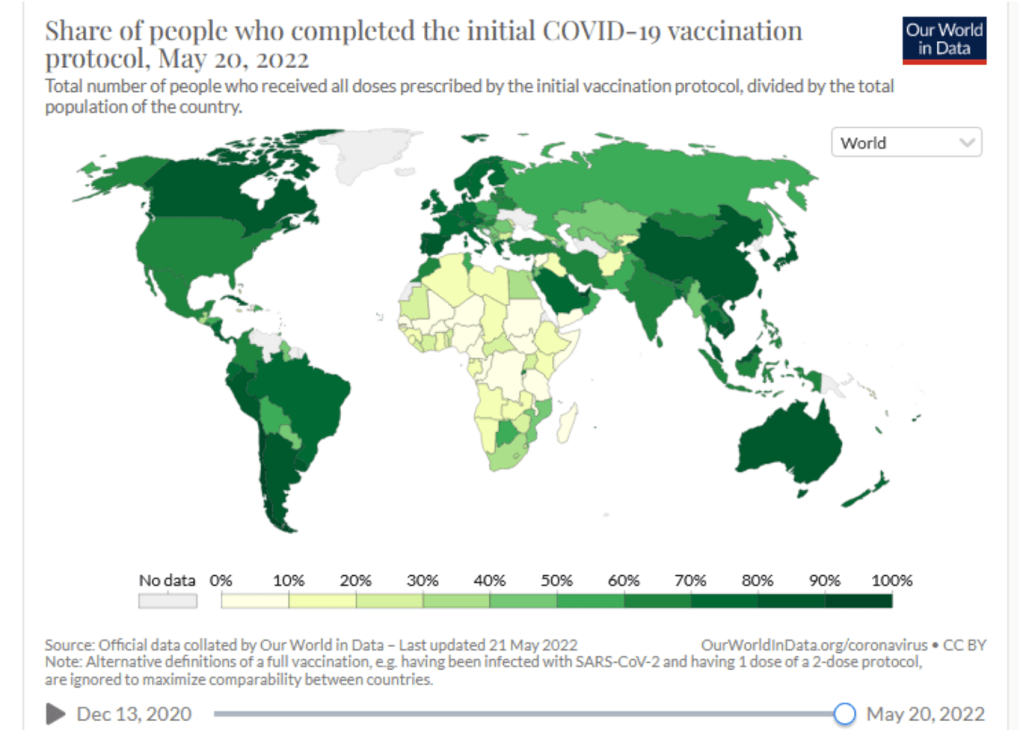
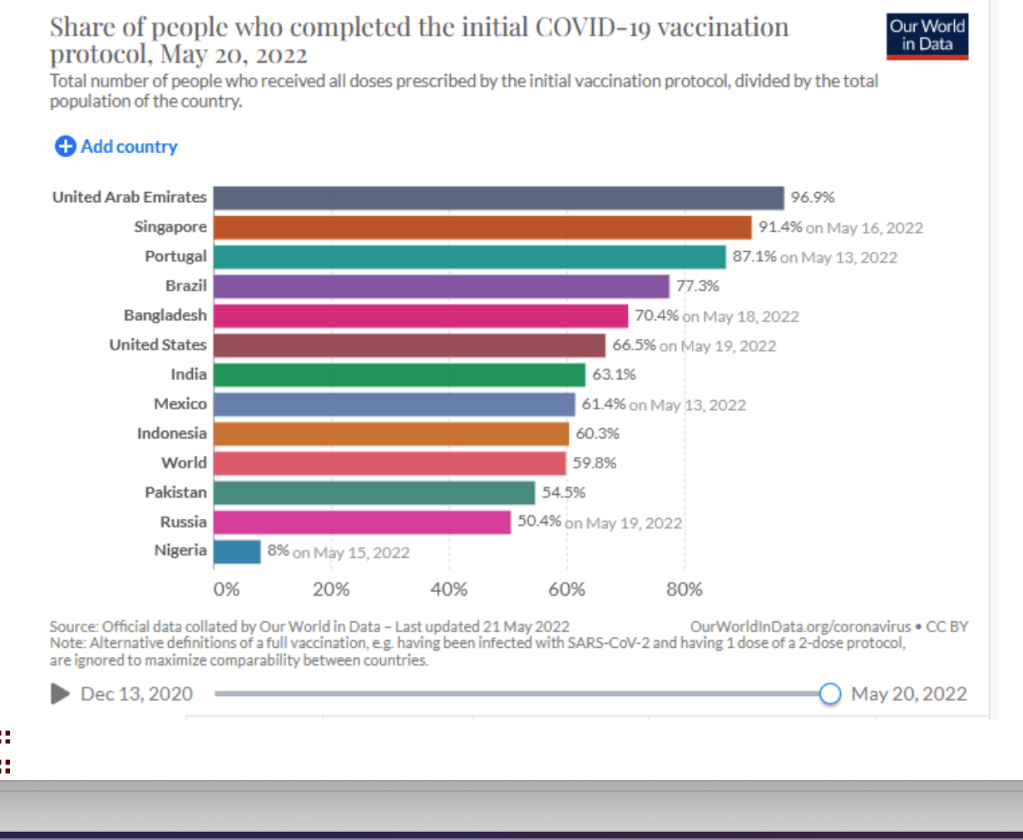
Only 16% of people in low-income countries have received a single vaccine dose – compared to 80% in high-income countries. In certain lower-income countries, many of the most at-risk people in society – healthcare workers, the elderly and those with underlying health conditions – are going unprotected while young, healthy adults receive booster doses in wealthier countries.
The world must act urgently to close this equity gap.
After a year of severe constraints, we are now in a situation that two years ago would have seemed impossible: namely global supply is now high enough to underpin the overarching objective of supporting equitable, full vaccination of all adult and adolescent populations globally. COVAX has access to more than enough doses needed to enable 91 lower-income countries that are supported by the COVAX Advance Market Commitment (AMC) – which provides donor-funded doses of a wide variety of COVID-19 vaccines – to meet their targets in light of the WHO global target of protecting 70% of the population in each country. We can support these countries to meet individual targets and prioritize full coverage of high-risk groups. In accordance with the updated SAGE roadmap of January 2022 – which recommends boosters for priority groups – COVAX is now open to and encouraging requests for doses for booster campaigns from countries.
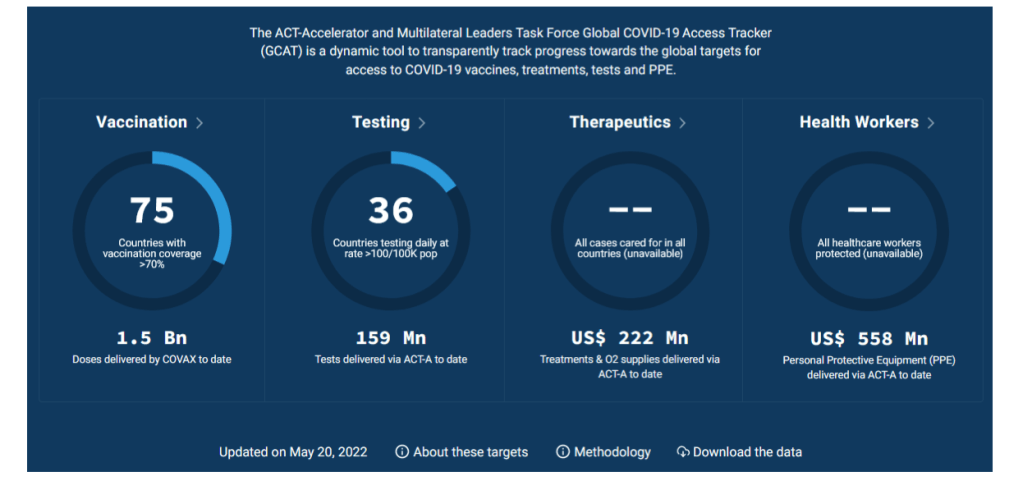
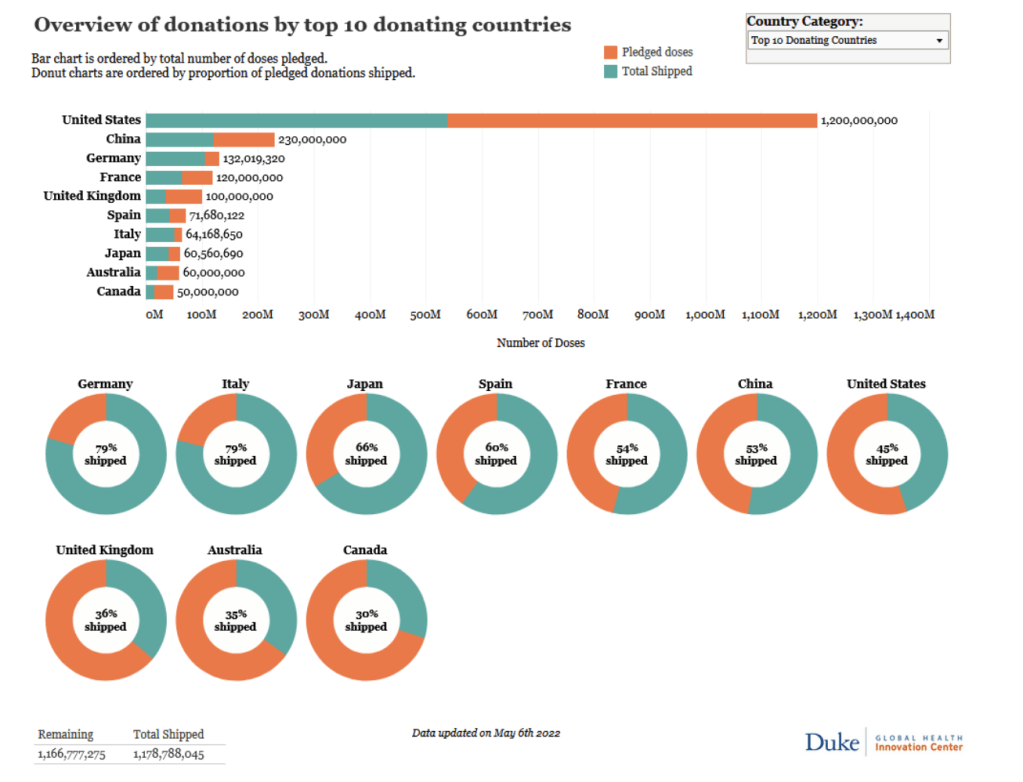
COVAX is also well-placed to deliver these doses so they reach those in need. In just 15 months, COVAX – as the Vaccines Pillar of the ACT-Accelerator partnership for equitable access to COVID-19 tools – has shipped over 1.3 billion vaccines to 87 low and lower-middle income countries around the world.
COVAX shipments account for 82% of vaccines delivered to low-income countries and the majority of COVID-19 vaccines administered in humanitarian settings. Through driving the fastest, largest and most complex global vaccination effort in history, COVAX’s work has helped raise the average proportion of people protected by a full course of vaccines in these low- and lower-middle income countries to 46%.
The onus now is on building on this foundation to help countries to fully protect high risk groups, meet national vaccination targets, and close the global COVID-19 vaccine equity gap for good. However, hurdles now remain: demand and uptake are low, with low-income countries remaining the furthest behind.
Demand and delivery
With more than 3.8 billion COVID-19 doses administered to-date, national governments in lower-income countries have led the way. The number of countries with coverage under 10% of the population has decreased from 34 in January to 18 today. Some AMC-supported countries – for example, Bhutan, Cambodia, Viet Nam, Maldives, Fiji and Bangladesh – have coverage above 70%.
Building on country readiness efforts undertaken so far, the countries furthest behind are receiving tailored support. In January 2022, WHO, UNICEF and Gavi established the COVID-19 Vaccine Delivery Partnership (CoVDP), an inter-agency initiative building on existing resources globally, regionally and in-country to support COVID-19 vaccine delivery in lower-income countries. CoVDP specifically provides urgent operational support to the 34 countries that were at or below 10% full vaccination coverage in January 2022, many of which are in Africa. By mid-April 2022, a total of $29 million of urgent funding was coordinated and disbursed – within 15 working days or less – to ten countries across the three agencies. The CoVDP has driven political engagement with several countries with low vaccination rates to keep vaccine delivery on top of the political agenda and identify opportunities for bundling COVID-19 vaccinations with other health interventions.
While CoVDP and other efforts are helping lower-income countries make progress, challenges still need to be addressed. In many countries Omicron has reduced the perceived risk of the virus and there are other health priorities that people and governments are focused on. The option of integrating COVID-19 vaccination with other health systems activities – for example, measles and polio campaigns, or the distribution of malaria bed nets, – therefore becomes more important. COVID-19 vaccination is also an opportunity to strengthen health systems including training health workers, enhancing health management information systems, further improving the cold chain, and forging new ways of working in fragile and conflict settings.
Through our continuous dialogue with AMC-supported countries, we know that demand is highly dynamic and difficult to predict, even for countries themselves. Thanks to input from national governments, COVAX is able to make some initial estimates of overall demand from AMC-supported countries. From our analysis of the latest demand planning forecasts provided to us by these countries, estimated country demand from now through early 2023 currently stands at approximately 330 million doses from COVAX, in addition to what has already been delivered or accepted by countries. However, these continue to be dynamic figures, and will evolve with situations on the ground as well as the occurrence of new variants. As such, COVAX will be working with national governments to continuously update these estimates.
In order for the world to continue to make meaningful progress on closing the global vaccine equity gap, we urgently call on countries to set ambitious targets backed by concrete plans for implementation – prioritising full coverage of high risk groups – and on all partners to coordinate on providing countries with the resources needed to accelerate and expand national strategies, stimulate demand and overcome operational bottlenecks. The next 3-4 months are crucial for accelerating COVID-19 vaccination campaigns, alongside moving to integrate COVID-19 vaccination efforts into routine primary health systems.
Supply
It is testament to the ground-breaking work of the scientific and manufacturing communities that there is now enough global supply to meet needs, as is the fact that it took only 327 days to go from the SARS-CoV-2 virus being sequenced and published to bringing a COVID-19 vaccine into emergency use.
As we strive to help countries deploy more doses, we must also work ensure supply remains available, at the right times. COVAX experienced delays securing doses in 2021. With many doses from advance purchase agreements now becoming available alongside donated doses, and the dynamic nature of country demand coupled with global oversupply, it is highly likely that overall supply will exceed demand. However, COVAX is working with manufacturers to help make supply more responsive to the changing demand environment.
That both global and COVAX supply now exceed demand is an advantageous situation in a pandemic, as it guarantees all countries long term availability of supply and choice of product. Protecting populations rapidly must now take priority. This is fundamental, given that 2021 clearly demonstrated the impact ad hoc, unpredictable supply had on the ability for countries with more limited health systems resources to plan and roll-out vaccination campaigns. Certainty of supply enables countries to plan national vaccination campaigns with more confidence, ensures a rolling buffer of stock can be available in-country and aids smooth and efficient roll outs.
While every effort should be taken to minimize wastage and expiry, lower income countries should also be able to accept doses, and to aim high, without being stigmatized when there is wastage, which is an inevitable part of immunization efforts against any disease, in any country.
COVAX is committed to providing countries with long-term, predictable supply, catering to all contexts and maintaining reserves to ensure supply can keep up with shifts in demand. This includes working with manufacturers and donors to ensure any new, variant-adapted vaccines reach COVAX at the same time as higher income countries.
We call on donor countries and manufacturers to support COVAX by ensuring the volume and timing of deliveries match as closely as possible the needs of lower-income countries. Donors should support COVAX in maintaining a diverse portfolio, including variant-adapted vaccines if necessary. Manufacturers should work with COVAX to re-phase or re-size supply from existing advance purchase agreements.
A world beset by numerous challenges and crises does not change the fact that the pandemic – our collective crisis – is far from over. Closing the vaccine equity gap must continue to be an urgent priority for the international community.