JAMA Network
COVID-19 Update March 19, 2022
These articles on COVID-19 were published across the JAMA Network in the last week.
JAMA Network
COVID-19 Update March 19, 2022
These articles on COVID-19 were published across the JAMA Network in the last week.
The Lancet
Mar 19, 2022 Volume 399 Number 10330 p1093-1200, e15-e16
https://www.thelancet.com/journals/lancet/issue/current
Editorial
Nigeria: rightly taking its place on the world stage
The Lancet
Nigeria is emerging as a world power. It has great intellectual, cultural, and social capital, as well as financial assets. It dominates west Africa, having more than half of the region’s population, and has the highest gross domestic product on the continent. The population of more than 200 million is projected to double by 2050, and to reach 733 million by 2100—making Nigeria the third most populous country in the world, after China and India. This rapid population growth has been accelerated by falling infant mortality combined with a steady birth rate and can create a demographic dividend for Nigeria. But to take advantage of this situation, appropriate investments in health, education, and skills need to be made. Published today, The Lancet Nigeria Commission: investing in health and the future of the nation, views this human potential and extraordinary opportunity through a health lens, telling the story of Nigeria as shaped by the country’s history and present circumstances. Written by a team of experts working at institutions across the country, and members of the diaspora, it has been led by Nigerians for Nigerians.
This potential might not be realised if the country does not address intractable poverty and extreme inequality. Recent trends in health outcomes, as detailed in the accompanying Article published today, record 20 years of increased healthy life expectancy (although it is still low within the region, at 56 years), reductions in mortality for males and females of all ages, and rises in health expenditure but, overall, health outcomes are still poor. Nigeria has repeatedly failed to realise the health gains promised by multiple political leaders, and this failure is holding the country back…
The Lancet
Mar 19, 2022 Volume 399 Number 10330 p1093-1200, e15-e16
https://www.thelancet.com/journals/lancet/issue/current
Articles
Population health outcomes in Nigeria compared with other west African countries, 1998–2019: a systematic analysis for the Global Burden of Disease Study
Blake Angell, at al
Open Access
The Lancet
Mar 19, 2022 Volume 399 Number 10330 p1093-1200, e15-e16
https://www.thelancet.com/journals/lancet/issue/current
Articles
Population health outcomes in Nigeria compared with other west African countries, 1998–2019: a systematic analysis for the Global Burden of Disease Study
Blake Angell, at al
Open Access
The Lancet
Mar 19, 2022 Volume 399 Number 10330 p1093-1200, e15-e16
https://www.thelancet.com/journals/lancet/issue/current
Effectiveness of the Ad26.COV2.S vaccine in health-care workers in South Africa (the Sisonke study): results from a single-arm, open-label, phase 3B, implementation study
Linda-Gail Bekker, et al. the Sisonke Protocol Team, on behalf of the Sisonke Study Team
The Lancet
Mar 19, 2022 Volume 399 Number 10330 p1093-1200, e15-e16
https://www.thelancet.com/journals/lancet/issue/current
The Lancet Commissions
The Lancet Nigeria Commission: investing in health and the future of the nation
Ibrahim Abubakar, et al.
Open Access
Nature
Volume 603 Issue 7901, 17 March 2022
https://www.nature.com/nature/volumes/603/issues/7901
Article | 31 January 2022 | Open Access
Vaccines elicit highly conserved cellular immunity to SARS-CoV-2 Omicron
Current vaccines induce broadly cross-reactive cellular immunity against SARS-CoV-2 variants, including Omicron, and provide protection against severe disease despite a substantially reduced neutralizing antibody response.
Jinyan Liu, Abishek Chandrashekar, Dan H. Barouch
Nature Biotechnology
Volume 40 Issue 3, March 2022
https://www.nature.com/nbt/volumes/40/issues/3
Patents | 16 March 2022
Contractual solutions to overcome drug scarcity during pandemics and epidemics
Licensing provisions that obligate recipients of government funding to share relevant technology and know-how for scarce drugs during pandemics and epidemics can reduce shortages and overcome obstacles that intellectual property rights present.
Sapna Kumar
Ana Santos Rutschman
Nature Biotechnology
Volume 40 Issue 3, March 2022
https://www.nature.com/nbt/volumes/40/issues/3
Article | 18 August 2021
Pre-activated antiviral innate immunity in the upper airways controls early SARS-CoV-2 infection in children
Single-cell sequencing reveals pre-activated immunity as important for milder COVID-19 symptoms in children.
J. Loske, J. Röhmel. I. Lehmann
Nature Genetics
Volume 54 Issue 3, March 2022
https://www.nature.com/ng/volumes/54/issues/3
Editorial | 14 March 2022
Rare diseases, common challenges
The genetics community has a particularly important part to play in accelerating rare disease research and contributing to improving diagnosis and treatment. Innovations in sequencing technology and machine learning approaches have positively affected diagnostic success, but more coordinated efforts are needed to move towards effective therapies or even cures for these important, and sometimes overlooked, class of diseases.
Nature Genetics
Volume 54 Issue 3, March 2022
https://www.nature.com/ng/volumes/54/issues/3
Comment | 07 March 2022
A call for an integrated approach to improve efficiency, equity and sustainability in rare disease research in the United States
To build a more efficient, equitable and sustainable approach to rare disease research in the United States, we must prioritize integrated research infrastructure and approaches that focus on understanding connections across rare diseases.
Meghan C. Halley, Hadley Stevens Smith, Holly K. Tabor
Nature Reviews Drug Discovery
Volume 21 Issue 3, March 2022
https://www.nature.com/nrd/volumes/21/issues/3
Comment | 28 January 2022
A call to action for translational sciences in COVID-19 and future pandemics
Translation Together, a transnational consortium of translational research organizations, reflects on successes and challenges in regional COVID-19 pandemic responses and proposes five priorities to improve preparedness for future global public health crises and improve the global approach to translational research and science.
Kanny K. Wan, Danielle Davis, Christine M. Cutillo
Nature Reviews Drug Discovery
Volume 21 Issue 3, March 2022
https://www.nature.com/nrd/volumes/21/issues/3
Comment | 28 January 2022
A call to action for translational sciences in COVID-19 and future pandemics
Translation Together, a transnational consortium of translational research organizations, reflects on successes and challenges in regional COVID-19 pandemic responses and proposes five priorities to improve preparedness for future global public health crises and improve the global approach to translational research and science.
Kanny K. Wan, Danielle Davis, Christine M. Cutillo
New England Journal of Medicine
March 17, 2022 Vol. 386 No. 11
https://www.nejm.org/toc/nejm/medical-journal
Perspective
A Malaria Vaccine for Africa — An Important Step in a Century-Long Quest P.L. Alonso and K.L. O’Brien
Malaria continues to devastate people’s health and livelihoods worldwide. In October 2021, the WHO for the first time recommended the large-scale use of a malaria vaccine for children living in areas with moderate-to-high malaria transmission.
Original Articles
New England Journal of Medicine
March 17, 2022 Vol. 386 No. 11
https://www.nejm.org/toc/nejm/medical-journal
Original Articles
Homologous and Heterologous Covid-19 Booster Vaccinations R.L. Atmar and Others
New England Journal of Medicine
March 17, 2022 Vol. 386 No. 11
https://www.nejm.org/toc/nejm/medical-journal
PLoS One
http://www.plosone.org/
[Accessed 19 Mar 2022]
Research Article
Use of oral polio vaccine and the incidence of COVID-19 in the world
Farrokh Habibzadeh, Konstantin Chumakov, Mohammad M. Sajadi, Mahboobeh Yadollahie, Kristen Stafford, Ashraf Simi, Shyamasundaran Kottilil, Iman Hafizi-Rastani, Robert C. Gallo
Research Article | published 17 Mar 2022 PLOS ONE
https://doi.org/10.1371/journal.pone.0265562
PLoS One
http://www.plosone.org/
[Accessed 19 Mar 2022]
The Vaccination Concerns in COVID-19 Scale (VaCCS): Development and validation
Kyra Hamilton, Martin S. Hagger
Research Article | published 14 Mar 2022 PLOS ONE
https://doi.org/10.1371/journal.pone.0264784
PNAS – Proceedings of the National Academy of Sciences of the United States
March 1, 2022 | vol. 119 | no. 9
https://www.pnas.org/toc/pnas/119/9
February 22, 2022 | vol. 119 | no. 8
https://www.pnas.org/toc/pnas/119/8
Research Article February 7, 202 2Open Access
Testing fractional doses of COVID-19 vaccines
Due to the enormous economic, health, and social costs of the COVID-19 pandemic, there are high expected social returns to investing in parallel in multiple approaches to accelerating vaccination. We argue there are high expected social returns to …
Witold Więcek, Amrita Ahuja,[…]Brandon Joel Tan
PNAS – Proceedings of the National Academy of Sciences of the United States
March 1, 2022 | vol. 119 | no. 9
https://www.pnas.org/toc/pnas/119/9
Research Article February 22, 2022 Open Access
Widespread use of National Academies consensus reports by the American public
In seeking to understand how to protect the public information sphere from corruption, researchers understandably focus on dysfunction. However, parts of the public information ecosystem function very well, and understanding this as well will help in …
Diana Hicks, Matteo Zullo,[…]Omar I. Asensio
Travel Medicine and Infectious Diseases
Volume 46 March–April 2022
https://www.sciencedirect.com/journal/travel-medicine-and-infectious-disease/vol/46/suppl/C
Review article Full text access
Travel vaccines throughout history
Androula Pavli, Helena C. Maltezou
Article 102278
Travel Medicine and Infectious Diseases
Volume 46 March–April 2022
https://www.sciencedirect.com/journal/travel-medicine-and-infectious-disease/vol/46/suppl/C
Research article Open access
Health and security risks of humanitarian aid workers during field missions: Experience of the International Red Cross
S.C. Guisolan, M. Ambrogi, A. Meeussen, F. Althaus, G. Eperon
Article 102275
Vaccine
Volume 40, Issue 9 Pages 1191-1384 (23 February 2022)
https://www.sciencedirect.com/journal/vaccine/vol/40/issue/9
Short communication Open access
Conditionality of COVID-19 vaccine acceptance in European countries
Leonardo W. Heyerdahl, Muriel Vray, Benedetta Lana, Nastassia Tvardik, … Tamara Giles-Vernick
Pages 1191-1197
Vaccine
Volume 40, Issue 9 Pages 1191-1384 (23 February 2022)
https://www.sciencedirect.com/journal/vaccine/vol/40/issue/9
Review article Open access
How to accelerate the supply of vaccines to all populations worldwide? Part I: Initial industry lessons learned and practical overarching proposals leveraging the COVID-19 situation
Mic McGoldrick, Thierry Gastineau, Diane Wilkinson, Cristiana Campa, … Samir Desai
Pages 1215-1222
Vaccine
Volume 40, Issue 9 Pages 1191-1384 (23 February 2022)
https://www.sciencedirect.com/journal/vaccine/vol/40/issue/9
Review article Open access
How to accelerate the supply of vaccines to all populations worldwide? Part II: Initial industry lessons learned and detailed technical reflections leveraging the COVID-19 situation
Mic McGoldrick, Thierry Gastineau, Diane Wilkinson, Cristiana Campa, … Samir Desai
Pages 1223-1230
Vaccine
Volume 40, Issue 9 Pages 1191-1384 (23 February 2022)
https://www.sciencedirect.com/journal/vaccine/vol/40/issue/9
Research article Full text access
COVID-19 vaccination intention and behavior in a large, diverse, U.S. refugee population
Jana Shaw, Kathryn B. Anderson, Rachel E. Fabi, Carlie A. Thompson, … Andrea V. Shaw
Pages 1231-1237
Vaccine
Volume 40, Issue 9 Pages 1191-1384 (23 February 2022)
https://www.sciencedirect.com/journal/vaccine/vol/40/issue/9
Research article Open access
Phase I interim results of a phase I/II study of the IgG-Fc fusion COVID-19 subunit vaccine, AKS-452
Yester F. Janssen, Eline A. Feitsma, Hendrikus H. Boersma, David G. Alleva, … Todd C. Zion
Pages 1253-1260
Vaccine
Volume 40, Issue 9 Pages 1191-1384 (23 February 2022)
https://www.sciencedirect.com/journal/vaccine/vol/40/issue/9
Research article Abstract only
Critical success factors for conducting human challenge trials for vaccine development in low- and middle-income countries
Keiko Pempho Msusa, Taryn Rogalski-Salter, Henshaw Mandi, Ralf Clemens
Pages 1261-1270
Vaccine
Volume 40, Issue 9 Pages 1191-1384 (23 February 2022)
https://www.sciencedirect.com/journal/vaccine/vol/40/issue/9
Research article Open access
Health and economic impact of seasonal influenza mass vaccination strategies in European settings: A mathematical modelling and cost-effectiveness analysis
Frank G. Sandmann, Edwin van Leeuwen, Sibylle Bernard-Stoecklin, Itziar Casado, … Marc Baguelin
Pages 1306-1315
Vaccine
Volume 40, Issue 9 Pages 1191-1384 (23 February 2022)
https://www.sciencedirect.com/journal/vaccine/vol/40/issue/9
Research article Abstract only
Pertussis burden and acellular pertussis vaccine effectiveness in high risk children
Sarah Sheridan, Peter McIntyre, Bette Liu, Parveen Fathima, … Heather Gidding
Pages 1376-1382
Think Tanks
Brookings
http://www.brookings.edu/
Accessed 19 Mar 2022
[No new digest content identified]
Center for Global Development [to 19 Mar 2022]
https://www.cgdev.org/
Publications [Selected]
March 15, 2022
Learning Loss and Student Dropouts during the COVID-19 Pandemic: A Review of the Evidence Two Years after Schools Shut Down
Following the outbreak and spread of COVID-19 in 2020, schools around the world closed for significant periods of time. Many scholars provided projections of the likely impacts on educational outcomes, with potentially dire impacts on learning loss and—especially in low-income contexts–dropout rates. Now, two years after schools began shutting down, we identify 40 empirical studies directly estimating student learning loss (29 studies) or dropout rates (15 studies) for students in pre-primary, primary, or secondary school in countries at any income level.
Laura Moscoviz and David Evans
Chatham House [to 19 Mar 2022]
https://www.chathamhouse.org/
Accessed 19 Mar 2022
[No new digest content identified]
CSIS
https://www.csis.org/
Accessed 19 Mar 2022
Podcast Episode
Live From Munich: Dr. Richard Hatchett “Pandemic Preparedness Needs to Be Viewed as a Security Challenge”
March 15, 2022 | By J. Stephen Morrison
Kaiser Family Foundation
https://www.kff.org/search/?post_type=press-release
Accessed 19 Mar 2022
March 16, 2022 News Release
States Are Planning for the End of the Continuous Enrollment Requirement in Medicaid After the COVID-19 Public Health Emergency Expires, But Many Have Not Made Key Decisions
As states plan for the end of the COVID-19 public health emergency, the resumption of eligibility redeterminations and disenrollments when the continuous Medicaid enrollment requirement is lifted could lead to coverage disruptions and losses, according to a new KFF 50-state survey. The requirement, a condition of states receiving enhanced federal…
March 15, 2022 News Release
Telehealth Continues to Account for More Than a Third of Outpatient Visits for Mental Health and Substance Use Services Well into the COVID-19 Pandemic
A new analysis from KFF and Epic Research finds that telehealth visits for outpatient mental health and substance use services went from virtually zero percent in 2019 prior to the COVID-19 pandemic to a peak of 40% in mid-2020 – and continued to account for more than a third (36%)…
Rand [to 19 Mar 2022]
https://www.rand.org/pubs.html
Reports, Selected Journal Articles
[No new digest content identified]
Selected General Media
The Economist
This week A special edition on our coronavirus coverage
March 19th 2022
China is enduring its first big outbreak of the Omicron variant. At least 40m people are under some form of lockdown (though the authorities in some regions prefer the term “life on pause”). The restrictions pose a threat to China’s economic rebound and the world’s supply chains.
In Hong Kong the death rate from covid-19 is twice as large as the peak of Britain’s second wave in early 2021. Morgues are overflowing with victims and hospitals are struggling to cope.
The latest outbreak has delayed any relaxation of China’s zero-covid policy. Our Chaguan column explains why beating covid on the mainland will require less fear, and more vaccinations.
African countries are also struggling to get people jabbed against covid. Just 13% of the continent has been fully vaccinated. Africa has plenty of covid doses but the increased deliveries are exposing logistical defects in the distribution of vaccines.
To end on a sliver of good news: covid has led to other vaccine victories. Our data-driven coverage this week shows how a jab against malaria is arriving at last. It could save as many lives as covid has taken. And in our Asia section, we look at changing attitudes towards the human papillomavirus vaccine in Japan as inoculations become more routine.
Vaccines and Global Health: The Week in Review is a weekly digest summarizing news, events, announcements, peer-reviewed articles and research in the global vaccine ethics and policy space. Content is aggregated from key governmental, NGO, international organization and industry sources, key peer-reviewed journals, and other media channels. This summary proceeds from the broad base of themes and issues monitored by the Center for Vaccine Ethics & Policy in its work: it is not intended to be exhaustive in its coverage. You are viewing the blog version of our weekly digest, typically comprised of between 30 and 40 posts below all dated with the current issue date
.– Request an Email Summary: Vaccines and Global Health : The Week in Review is published as a single email summary, scheduled for release each Saturday evening before midnight (EDT in the U.S.). If you would like to receive the email version, please send your request to david.r.curry@centerforvaccineethicsandpolicy.org.
– pdf version: A pdf of the current issue is available here:
– blog edition: comprised of the approx. 35+ entries posted below.
– Twitter: Readers can also follow developments on twitter: @vaxethicspolicy.
.
– Links: We endeavor to test each link as we incorporate it into any post, but recognize that some links may become “stale” as publications and websites reorganize content over time. We apologize in advance for any links that may not be operative. We believe the contextual information in a given post should allow retrieval, but please contact us as above for assistance if necessary.
Support this knowledge-sharing service: Your financial support helps us cover our costs and to address a current shortfall in our annual operating budget. Click here to donate and thank you in advance for your contribution.
.
David R. Curry, MS
Executive Director
Center for Vaccine Ethics and Policy
Global community comes together in support of 100 Days Mission and pledges over $1.5 billion for CEPI’s pandemic-busting plan
OSLO, Norway, 8 March 2022: The global community today came together to commit to the 100 Days Mission – the ambition to have safe and effective vaccines within 100 days of an epidemic or pandemic threat being identified – and pledged $1.535 billion to the Coalition for Epidemic Preparedness Innovations (CEPI) to help kick start the organization’s ambitious plan to tackle epidemics and pandemics, potentially saving millions of lives and trillions of dollars in lost economic output.
The pledges of political and financial support were made at the Global Pandemic Preparedness Summit, co-hosted by the UK Government, in London on 7-8 March 2022. Representatives from over 20 countries joined leaders from international agencies, science and academia, industry, philanthropy, and civil society to galvanize action around pandemic preparedness, and build momentum for the fundraising effort for CEPI’s five-year plan…
A GLOBAL PARTNERSHIP TO ACHIEVE THE 100-DAY MISSION AND STRENGTHEN PANDEMIC PREPAREDNESS WITH EQUITY AT ITS HEART
The Global Pandemic Preparedness Summit marks a landmark moment in the global community’s collective efforts to build a world that is better prepared to respond to epidemic and pandemic threats. Leading figures came together to share their insights and perspectives on COVID-19 and other infectious diseases such as Ebola and Lassa fever, to celebrate the innovations developed in response to the pandemic, to highlight the importance of access-focused R&D as a cornerstone of global health security, and to explore how to boost vaccine manufacturing across the world.
In addition to the funding pledged by investors, several important developments were announced on the day of the Summit which will contribute to CEPI’s plan to advance global pandemic preparedness and response:
– The UK Government, CEPI and representatives of the life sciences industry published a joint statement outlining their collective commitment to delivering on the 100 Days Mission
– UK Prime Minister Boris Johnson welcomed a new partnership between CEPI and Cambridge, UK based company DioSynVax to develop a new vaccine that could provide protection against a broad range of coronaviruses. CEPI will invest up to £31m ($42m) to support the development of the vaccine. This is the 7th award made by CEPI in pursuit of a broadly protective coronavirus vaccine
– CEPI and the United States National Institute of Allergy and Infectious Diseases (NIAID) – the two leading global funders of research into broadly protective coronavirus vaccines – will collaborate to establish a joint scientific forum for awardees who are developing pan-coronavirus vaccines to share scientific progress and challenges, with the aim of accelerating their progress. Eminent global vaccine experts Dr Barney Graham and Dr Peter Paradiso will serve as co-chairs of the first in its kind collaboration between the two organizations.
– CEPI and the Rwanda Biomedical Centre (RBC), a specialized technical institute of Rwanda, signed a Memorandum of Understanding to formalize a collaboration which aims to improve and accelerate vaccine research, development, and manufacturing in Rwanda. The MoU was signed while the Honourable Dr Daniel Ngamije Minister of Health for Rwanda was attending the Summit.
– Moderna announced its global health strategy, including a commitment to advance vaccines targeting 15 pathogens identified as the biggest public health risk by WHO and CEPI into clinical studies by 2025
– Aspen Pharmacare announced a landmark agreement with Johnson & Johnson to manufacture and make available a COVID-19 vaccine throughout Africa…
GLOBAL FINANCIAL COMMITMENTS
The UK Government pledged £160 million (US$211 million) to CEPI. Other top donors included the governments of Japan, Norway, USA, Germany, Australia, the Bill & Melinda Gates Foundation and Wellcome. G20 President Indonesia reaffirmed its support for CEPI with a pledge of $5m. Additional pledges were made at the Summit, and further commitments and donations are expected to follow in the coming months.
…Dr Richard Hatchett, CEO of CEPI, said: “Today’s Global Pandemic Preparedness Summit represents an important moment for the global health community to come together to meet the challenges of this and future pandemics. We are extremely grateful to our investors, supporters and partners for their visionary contribution to CEPI’s work.
“For the first time in history, we have the tools we need to eliminate the risk of future pandemics. It is vital that we capitalise on the scientific developments we’ve seen over the last two years and seize the rare alignment of political will, practical experience, and technical and scientific progress emerging from the pandemic to prevent such devastation happening again. The support and funding pledged today will kick-start CEPI’s plan to end pandemics. This is the start of an incredibly important journey.”
Eighth Meeting of the Multilateral Leaders Task Force on COVID-19, 1 March 2022: “Third Consultation with the CEOs of leading vaccine manufacturers” Joint Statement
WASHINGTON, March 7, 2022 —
The heads of the International Monetary Fund, World Bank Group, World Health Organization, and World Trade Organization held high-level consultations with UNICEF, Gavi, the Vaccine Alliance, the Global Lead Coordinator for the COVID-19 Vaccine Country Readiness and Delivery and the CEOs of leading vaccine manufacturers on 1 March 2022 aimed at ensuring the rapid delivery of vaccines to where they are needed the most and putting those vaccines into arms.
The Multilateral Leaders Task Force issued the following statement:
“In the past few months, we have seen unprecedented levels of disease transmission across the world due to the Omicron variant. Still, unequal access to COVID-19 vaccines, tests and treatments is rampant, prolonging the pandemic. 23 countries are yet to fully vaccinate 10% of their populations, 73 countries are yet to achieve 40% coverage and many more are projected to miss the 70% target by middle of this year.
The biggest challenges are in low-income countries (LICs), which are concentrated in Africa. Only 7% of people in LICs have been fully vaccinated, compared with 73% in high-income countries. Safeguarding the health of people living in the world’s poorest countries in the face of a changing pandemic is a key priority. We must and can ensure that these countries have the access, the means, and the capacity to vaccinate their populations, especially those who are most at risk.
Despite the challenges, there has been progress. The vaccine supply constraints from last year have eased, and export restrictions are not currently an issue. Our efforts must now focus on supporting countries to increase vaccination rates. There is no “one-size-fits-all” approach as each country faces different political, administrative, and capacity challenges.
Insufficient health care infrastructure, including warehouses, cold chain capacity; lack of trained vaccinators; complexities associated with the management of multiple vaccines; lack of data systems to support vaccination campaigns; and misinformation and vaccine hesitancy are common hurdles that governments must confront. But we have good lessons to draw on from countries around the world that have managed to overcome obstacles and rollout vaccination campaigns, including from low-income countries.
Sustained investment in geographically diversified manufacturing capacity and new technologies for vaccines, therapeutics, and diagnostics is key for ensuring more equitable, affordable, and timely access to tools for developing countries. In this context, we welcome the work of the leading vaccine manufacturers in exploring and undertaking new partnerships and call for them to work closely with international organizations (IOs) and countries to capitalize on practical solutions, sharing licenses, technology and know-how.
A top priority to end the pandemic is deploying financing quickly to accelerate the development, production, and equitable access to COVID-19 tests, treatments and vaccines in low- and middle-income countries. Fully funding the Access to COVID-19 Tools (ACT) Accelerator is critical.
As vaccine supply increases in 2022, close coordination among all stakeholders will be crucial to aligning supply with demand, reducing supply fragmentation, and deploying vaccines in the most effective way. We must adjust to constantly evolving challenges and keep working together. As the late Dr. Paul Farmer said: “Any time there’s a new tool developed – whether they are vaccines or therapeutics – there must also be a delivery plan.”
Let us acknowledge the importance of delivery, as this is where lives are saved, families are kept whole, children continue their education, communities stay strong, and economies grow.”
COVAX AMC Summit
Germany to co-host 2022 Gavi COVAX AMC Summit, pledges additional funding for COVID-19 vaccination in lower-income countries
Germany will co-host the 2022 AMC Summit to help raise urgent funding to support lower-income countries’ dynamic COVID-19 vaccination needs
In addition, Germany has pledged an additional EUR 350 million to Gavi for the COVAX AMC, as part of a broader US$1.22 billion funding package for the ACT-Accelerator, pending cabinet and parliamentary approval.
Both the Summit and pledge are in support of Germany’s G7 Presidency goal to promote healthy lives worldwide, strengthen efforts to address COVID-19 on a global scale, and prepare for future pandemics and health crises.
Prof. José Manuel Barroso, Board Chair of Gavi, the Vaccine Alliance: “Germany’s support for global vaccination and the fight against COVID-19 has been clear from the beginning. We thank the German government in their G7 Presidency year for their support in helping to break the COVID-19 pandemic by hosting this summit and pledging additional funding to the Gavi COVAX AMC and ACT-Accelerator. We particularly recognize and value that this leadership comes at a time when Berlin along with many other capitals are mobilizing to respond to the terrible civilian suffering created by the conflict in Ukraine.”
Svenja Schulze, German Minister for Economic Cooperation and Development: “To be agile and flexible in our response, we need sufficient resources – this is one of the key lessons learned in 2021 and essential to pandemic preparedness. We need to respond quickly to in-country needs and invest in strengthening country delivery systems in order to put an end to this pandemic. No one is safe until everyone is safe.”
Geneva, 11 March 2022 – Gavi, the Vaccine Alliance announced today that the Government of Germany will host a leader-level meeting aimed at helping raise at least US$5.2 billion in urgent financial support for COVAX, including US$ 3.8 billion in donor funding for lower-income countries supported by the Gavi COVAX Advance Market Commitment (Gavi COVAX AMC). The virtual event – “2022 Gavi COVAX AMC Summit: Break COVID Now” – will be co-chaired by Svenja Schulze, German Minister for Economic Cooperation and Development and José Manuel Barroso, Gavi Board Chair. It will take place on 8 April 2022…
…Hundreds of millions of people, mainly in lower-income countries, remain unvaccinated and unprotected, while the virus continues to evolve in uncertain ways: a major variant has been identified every 4 months, on average, since the pandemic was declared.
In 2022, COVAX will be focused on building on the foundation in place to tackle those challenges, serving as an adaptable and flexible mechanism to support lower-income countries’ national COVID-19 vaccine objectives, and contributing to the global effort to break COVID. That will mean providing urgent delivery support to those furthest behind in coverage – while closely monitoring each country’s continually evolving needs and strategies, including the need to prioritise and sequence COVID-19 immunization alongside routine immunization and other essential health systems activities. It will also mean learning from the lessons of the past year and making sure funding is available now to support countries’ needs in the face of inevitable future evolutions of the pandemic. To support these goals, COVAX is seeking urgent additional funding of at least US$5.2 billion, of which Gavi is seeking to raise US$3.8 billion from sovereign and private donors.
Catalytic delivery funding will help countries increase rates of administration, now that supply is in place, and there is short and medium-term visibility of supply for the first time in the 12 months that vaccines have been available to AMC participants. In response to the high demand to-date for the $900m COVID-19 Delivery Support package that Gavi and partners have already put in place, urgent additional funding of US$ 1 billion will help lower-income countries rapidly protect more people against COVID-19. Of this amount, Gavi is seeking US$ 600 million to build on its Alliance work and provide direct support to lower-income governments for a range of readiness activities, while UNICEF is seeking US$400 million to support the critical work of its offices in lower-middle income countries and humanitarian contexts. Germany has also committed to work towards this effort with additional funding.
While seeking to build on the progress made, however, it is equally critical that we do not squander the lead we finally have in terms of supply meeting demand. COVAX’s current portfolio can meet current demand, but this situation will not remain static. It is essential that we continue to mitigate and guard against future risks: variant-adapted vaccines, new vaccines, changes in booster policies or target populations, shift towards an annual shot model are all potential scenarios that the world must account for. Financing will need to already be in place to respond quickly in each scenario. Failing to do so will result in repeating the past, with lower-income countries once again being at the back of the queue.
To avoid such a situation and manage those risks, Gavi is seeking urgent funding for a “Pandemic Vaccine Pool” – a flexible financial instrument that blends direct, contingent and innovative financing and will be able to act as a rapid response mechanism to support lower-income countries’ needs in the face of these inevitable changes. Alongside US$ 2.7 billion in funding from sovereign and private sector donors, multilateral development banks and lower-income countries can also contribute via cost-sharing, providing another source of rapid funding for vaccines made available through the pandemic vaccine pool to the COVAX AMC.
Gavi is also seeking US$545 million to fund ancillary costs of dose donations, buying syringes and paying for logistics to get doses to countries, helping donations continue to be a sustainable and complementary source of supply alongside funded doses from COVAX agreements…
…“COVAX will need additional support through 2022 to ensure we can act now to support readiness and delivery to continue to accelerate rollout in countries, and anticipate and address future risks rapidly to meet future country demand. We must hope for the best, and plan for the worst: COVAX cannot again be at the back of the queue,” added Dr. Seth Berkley, CEO of Gavi, the Vaccine Alliance. “We look forward to bringing countries, manufacturers, donors, civil society and the private sector together on April 8th to strengthen the world’s collective response to COVID and commitment to future pandemic preparedness – and thank Germany for helping lead this effort.”
OHCHR Session – Resolution 46/14
Office of the High Commissioner for Human Rights
IFPMA Statement delivered at Panel Discussion on Universal Access to Vaccines – 20th meeting, 49th Regular
Statement from Mr. Thomas Cueni, Director General of IFPMA, in the Panel discussion on ensuring equitable, affordable, timely and universal access for all countries to vaccines in response to the COVID-19 pandemic (March 10th, 2022)
Excellencies, Chairperson,
The International Federation of Pharmaceutical Manufacturers & Associations (IFPMA) would like to thank the OHCHR for convening this half-day panel discussion in line with the resolution 46/14.
Being aware of the responsibility our industry has to patients and society, pharma companies large and small, in developing and developed countries have engaged, since the first days of the pandemic, in unprecedented levels of collaboration to find solutions to COVID-19. Industry stepped up, bringing its knowledge and expertise in the discovery and development of novel therapeutics and vaccines and in building manufacturing capacity and distribution networks. And yet, a large part of the eligible population in many African nations remains unvaccinated.
Some aspects of our collective efforts in mounting a response have been successful. We have witnessed the fastest vaccine development ever, in just under a year. Within less than three months of the first vaccine getting emergency use approval by the World Health Organization (WHO) on 31 December 2020, we have seen COVID-19 vaccines reaching people across the world.
A commitment to work together to achieve fair and equitable access was also forged in the early days of the pandemic, with the creation of a unique global public-private partnership, Access to COVID-19 Tools (ACT) Accelerator, which IFPMA joined as founding partner. The vaccine pillar COVAX has been an important vehicle for vaccine equity, with its first billion vaccine doses delivered end of January. Innovative biopharmaceutical companies have supplied four out of five of these vaccines, and more than 80 per cent of the COVAX vaccines have been supplied by our member companies. In addition to COVAX, over 3 billion doses, have been delivered to low- and lower-middle-income countries through bilateral or regional arrangements. Today, over 12 billion doses of vaccines have been produced – trebling pre-pandemic vaccine capacity – and more than 60 percent of the world’s population have received at least one dose.
The record speed development and successful scaling up of COVID-19 vaccine manufacturing was a remarkable triumph of science and engineering. We have seen bumps and glitches, vaccine projects in vaccine development and scaling up of manufacturing, including challenges faced due to export restrictions and bans by a number of countries. However, we made it and most public health experts today accept that the challenge is no longer the lack of vaccines, but the need for more vaccinations. Significant number of doses will continue to be delivered to lower-income countries, but at present we see that supply is exceeding absorption capacity in many countries.
But despite these successes, collectively, and this includes the biopharmaceutical industry, we have not managed to meet our ambitions of vaccine equity. The global challenge we now face to guarantee a widespread access to vaccines has turned from one concerning supply constraints to one regarding the ability to quickly administer the vaccines. To turn vaccines into vaccinations, and ensure that vaccines shipped effectively reach populations, attention is urgently needed on concrete measures in recipient countries to support COVID-19 vaccine deployment and uptake.
Manufacturers, governments, international institutions, and other non-governmental organizations must work together and redouble efforts now to support countries as they mobilize to execute national vaccine rollouts and remove barriers to the efficient distribution and administration of vaccine doses, so that they reach those who need them most.
To urgently support this endeavor, innovative biopharmaceutical companies will continue to work with all relevant stakeholders on the following three overarching priorities and supporting activities:
1) Step up support for country readiness to roll out COVID-19 vaccine doses
2) Contribute to equitable distribution of COVID-19 vaccine doses
3) Continue to drive innovation
As access to all who need the vaccines remains a fundamental issue that needs urgently addressing; but the focus on how intellectual property contributes to this inequity are misplaced and are not backed up by what has happened over the past two years.
The strength of a robust innovation ecosystem in response to the pandemic has been unprecedented and more than proven its effectiveness: driving innovation, underpinning partnerships, facilitating knowledge-sharing, and voluntary licensing, and technology transfer. The positive from this pandemic is the geographic diversity of vaccine manufacturing agreements that have been formed through voluntary collaborations amongst trusted partners, including via 370 collaborations across all five continents. Big companies with small biotechs, developing manufacturers with established ones. IP incentives are enabling the current R&D to combat variants, ongoing technology transfer and voluntary collaborations.
In addition to the technology transfer agreements in South Africa, put in place in the first days of the pandemic between for example Aspen Pharma and J&J, and Pfizer together with BioVac, new solutions are now being set up in other parts of the Sub-Saharan Africa. For example, BioNTech has announced it will establish its first manufacturing facility in the African Union in mid-2022 and expects to ship the modular production units to Rwanda and Senegal. Moderna has signed an agreement to build a manufacturing plant, with a planned capacity of 500 million doses, in Kenya; and has pledged to invest in R&D on 15 CEPI priority pathogens with aim to develop a ‘base’ vaccine against each of these pathogens.
Flexibility and voluntary solutions to collaborate have been and should continue to be a fundamental pillar to address future healthcare challenges.
To ensure that no-one is left behind in the race to tackle the pandemic which now enters its third year, the biopharmaceutical industry is fully committed to effective international cooperation focusing on getting vaccines from the tarmac into arms is what’s needed now more than ever.
Featured Analysis
WHO – Emerging trends and technologies: a horizon scan for global public health
11 March 2022
Overview
This publication presents the findings of a global horizon scan, conducted by a group of international experts, on emerging technologies and trends relevant to global public health conducted in 2020 and 2021. The group identified 15 new and emerging technologies and scientific advances that may have a significant impact on global health over the next two decades.
Advances in science and technology hold great promise and hope for new and improved ways to address global health and ensure healthier populations worldwide. They could potentially fundamentally transform global health. This horizon scan presents 15 priority topics, including ethical and societal challenges, and risks and opportunities that require closer attention.
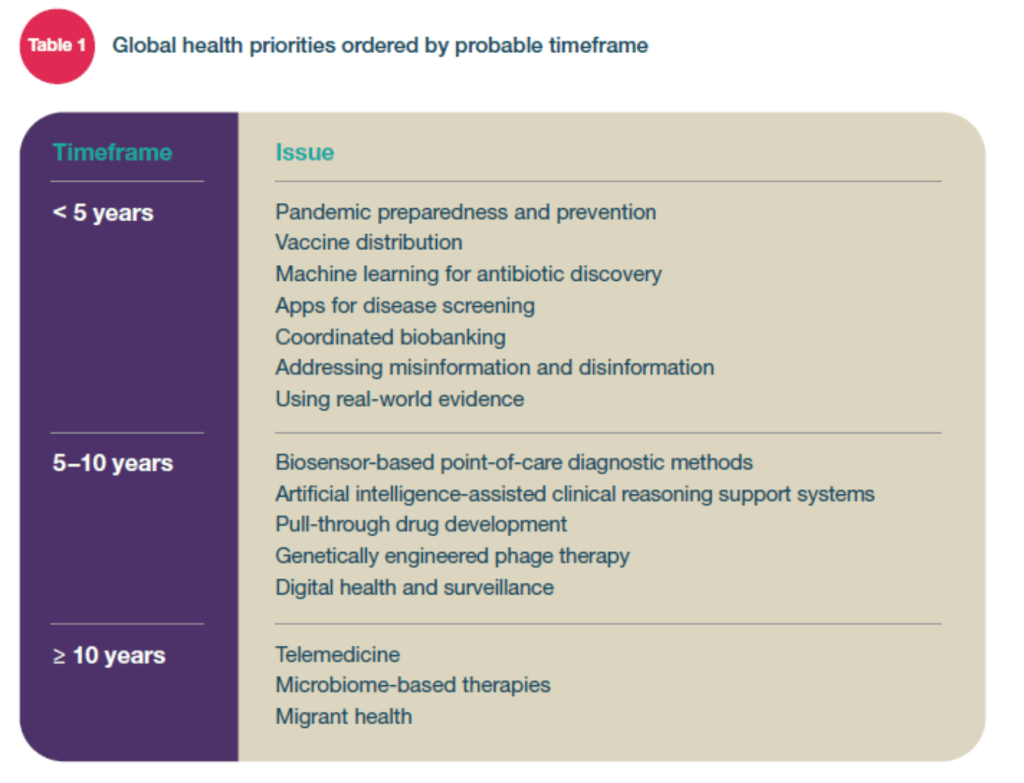
Issues identified to become prominent within 5 years
[Examples from list above]
Pandemic preparedness and prevention
A response to the COVID-19 pandemic requires a significant shift in resources and attention towards pandemic preparedness, particularly for zoonotic infections. Pandemics are likely to become more complex, frequent and difficult to contain for various reasons, including climate change, urbanization and interconnectivity (6).
An area for significant improvement in pandemic preparedness and response is trials of therapeutic interventions. A recent review by the US Food and Drug Administration (7) indicates that only about 5% of almost 2900 trial arms for potential COVID-19 therapeutics (involving > 500 000 patients) could be considered both randomized and adequately powered. Most of the studies could not provide useful results for the pandemic. Adaptive platform trials, in which multiple interventions are studied continuously, offer a promising way forward (8, 9).
As finance and policy shift towards infectious disease, efforts must be made to ensure that coordinated multilateral action prevails over national unilateralism. The Seventy-fourth World Health Assembly recommended a timely start to negotiation of an international treaty on pandemic preparedness and response. On 1 September 2021, the WHO Hub for Pandemic and Epidemic Intelligence was inaugurated in Berlin, Germany, and on 1 December 2021 a Special Session of World Health Assembly agreed by consensus to start a global process to draft and negotiate a convention, agreement or other international instrument under the Constitution of the World Health Organization to strengthen pandemic prevention, preparedness and response.
Vaccine distribution
More coordinated, effective systems of vaccine production and global distribution will be necessary both in the coming years, as COVID-19 vaccination programmes unfold, and in the longer term, as countries prepare for future disease outbreaks. During COVID-19, wide inequity in vaccination distribution have become apparent, ranging from “vaccine nationalism”, whereby countries prioritize their own populations over the global good, to “vaccine diplomacy”, whereby countries strategically
provide vaccines to others for geopolitical ends. Targeted, equitable, efficient distribution of vaccines would contain the pandemic sooner, lower global morbidity and mortality rates and reduce the chance of new strains arising (10).
Improving vaccine access will require coordination and significant changes to current approaches, not only extending vaccine production. Vaccines must also be accessible, affordable, trusted and used efficiently (10). Achievement of each of these criteria will require a cooperative global approach…
Conclusions [p.14]
[Excerpts]
In this horizon scan, we identified various technical, societal and economic issues that deserve close attention. WHO is already addressing a number of these areas and is actively engaged, for example, by convening expert groups, issuing guidelines and guidance and setting norms and standards on many of the topics identified…
…Proactive engagement must include not only critical assessment of the ethical dimensions, such as misuse, but also issues of access and equity. Equally important is building robust capacity and deploying resources to promote benefits and confront challenges arising from advances in science and emerging technologies with relevant skills to assist and inform the work of WHO, its Member States and stakeholders.
USA – AU/Africa CDC
FACT SHEET: United States Partnership with the African Union – Africa Centres for Disease Control and Prevention
March 11, 2022 • Statements and Releases
Today, the United States and African Union (AU) – Africa Centres for Disease Control and Prevention (Africa CDC) renewed our partnership by signing an updated Memorandum of Cooperation (MOC) between His Excellency Moussa Faki Mahamat, Chairperson of the African Union Commission (AUC), and U.S. Secretary of State Antony Blinken. This strategic memorandum signifies a continued and expanded commitment to this partnership by outlining our shared priorities for a safer, healthier, and more equitable future. Together, we will continue efforts to achieve the Sustainable Development Agenda.
At the February 2022 AU Heads of State Summit, a resolution was approved to elevate Africa CDC’s status to an autonomous health agency of the African Union – a recognition of its global and regional leadership during the COVID-19 pandemic, Ebola outbreaks, and many other health events. In its support to Member States, Africa CDC serves as a platform to share and exchange knowledge and lessons from public health interventions. Since our partnership officially began in 2015, the U.S. government and Africa CDC have worked together to combat some of the world’s most pressing crises, including Ebola and COVID-19. We have continued to build on our strong relationship through technical assistance, embedded experts and deployed staff to work alongside the organization, and funding to support Africa CDC activities and staffing.
Today, we celebrate ongoing work and highlight key areas of future engagement, including:
National Public Health Institutes (NPHIs): National public health institutes (NPHIs) serve as a functional home for public health activities and the foundation of Africa CDC’s operating model. These science-based agencies monitor the population’s health and respond to disease outbreaks and other public health priorities. They provide leadership and coordination to focus on core public health functions, including surveillance, laboratory, workforce, and public health emergency management. There is natural synergy between Africa CDC and the United States in establishing NPHIs and strengthening their core functions.
The United States has also supported Africa CDC’s three-tiered operational model by developing and strengthening NPHIs at the national level and at five Regional Collaborating Centers (RCCs). The RCCs help build capacity of, coordinate public health initiatives with, and coordinate resources among AU Member States. Current Africa CDC initiatives, in partnership with the United States, can address capacity needs of NPHIs, including surveillance networks, health information exchange, early warning systems, and development of testing and diagnostics capacity. Our partnership will help support Africa CDC’s ongoing efforts to expand health security throughout the continent, and help countries reach the goals and target of the Global Health Security Agenda.
Public Health Workforce: The United States and Africa CDC share the perspective that the public health and healthcare workforce plays a critical role in strengthen health systems, including preparing for and responding to pandemics while continuing to address health needs. The United States works with Africa CDC in building Africa’s public health workforce through supporting global human resources for health, expanding the Field Epidemiology Training Program (FETP), and partnering with Africa CDC’s Institute for Workforce Development (IWD) on other capacity building efforts.
COVID-19: As part of President Biden’s commitment to leading an international and coordinated vaccine effort, the United States has shared over 166 million COVID-19 vaccine doses with 48 countries in Africa, as of February 2022, with plans for sending more. Africa CDC, in partnership with the African Union, the African Vaccine Acquisition Trust and COVAX, has helped guide allocations of those doses. The United States is supporting updates to regulatory structures and is facilitating engagement with private sector and international organizations that will help expand vaccine manufacturing and enable African public health institutions to produce and supply vaccines for the continent. The U.S. government has partnered with Africa CDC to enhance surveillance and laboratory capacity to identify, detect, and respond to COVID-19 outbreaks and variants of concern. The United States has also deployed staff to support and collaborate with Africa CDC in its leadership of COVID-19 response in Africa.
Critical Medical Commodities: Through the United States Government’s Initiative for Global Vaccine Access (Global VAX), we are expanding efforts to work with international partners like Africa CDC to support infrastructure and capacity in vaccine delivery. While we currently work together on COVID-19 vaccine development, these efforts lay the foundation for accelerating the development of vaccines, therapeutics, and other critical medicals tools for a range of diseases. This complements existing work and technical partnerships across Africa for similar efforts to meet immediate and long-term health needs.
Advanced Molecular Detection: Advanced technologies such as next-generation sequencing and bioinformatics are essential public health tools to help prevent and respond to emerging health threats. Genomics is an increasingly important tool in emerging potential in global public health. The United States, Africa CDC, and other partners have worked together to launch Africa CDC’s Pathogen Genomics initiative to strengthen laboratory systems and enhance disease surveillance by equipping the continent’s public health institutions with the tools, training, and data infrastructure to fully leverage critical genomic sequencing technologies.
SARS-CoV-2 in wildlife/animal reservoirs
Joint statement on the prioritization of monitoring SARS-CoV-2 infection in wildlife and preventing the formation of animal reservoirs
7 March 2022
Joint Statement by Food and Agriculture Organization (FAO), World Organisation for Animal Health (OIE) and World Health Organization (WHO)
As we enter the third year of the pandemic, SARS-CoV-2, the virus that causes COVID-19, is spreading between people at an intense level globally. There are many factors that are driving transmission. One of these is the emergence of highly transmissible variants of concern, the latest being Omicron. The virus continues to evolve and the risk of future emergence of variants is high.
Although the COVID-19 pandemic is driven by human-to-human transmission, the SARS-CoV-2 virus is also known to infect animal species. Current knowledge indicates that wildlife does not play a significant role in the spread of SARS-CoV-2 in humans, but spread in animal populations can affect the health of these populations and may facilitate the emergence of new virus variants.
In addition to domestic animals, free-ranging, captive or farmed wild animals such as big cats, minks, ferrets, North American white-tailed deer and great apes have thus far been observed to be infected with SARS-CoV-2. To date, farmed mink and pet hamsters have been shown to be capable of infecting humans with the SARS-CoV-2 virus and a potential case of transmission between white-tailed deer and a human is currently under review.
The introduction of SARS-CoV-2 to wildlife could result in the establishment of animal reservoirs. For example, it has been reported that, approximately one-third of wild white-tailed deer in the United States of America have been infected with SARS-CoV-2, initially via several human-to-deer transmission events. The SARS-CoV-2 lineages detected in white-tailed deer have also been circulating in close-by human populations. White-tailed deer have been shown to shed virus and transmit it between each other.
FAO, OIE and WHO call on all countries to take steps to reduce the risk of SARS-CoV-2 transmission between humans and wildlife with the aim of reducing the risk of variant emergence and for protecting both humans and wildlife. We urge authorities to adopt relevant regulations and disseminate previously released recommendations by FAO, OIE and WHO to (1) people working in close contact with or handling wildlife, including hunters and butchers; and (2) the public.
Personnel working closely with wildlife should be trained to implement measures that reduce the risk of transmission between people and between people and animals, using WHO advice on how to protect oneself and prevent the spread of COVID-19, and OIE and FAO guidelines on the use of personal protective equipment (PPE) and good hygiene practices around animals, including good hygiene practices for hunters and butchers .
Current evidence suggests that humans are not infected with the SARS-CoV-2 virus by eating meat. However, hunters should not track animals that appear sick or harvest those that are found dead. Appropriate butchering and food preparing techniques, including proper hygiene practices, can limit transmission of coronaviruses, including SARS-CoV-2, and other zoonotic pathogens.
FAO, OIE and WHO stress that the public should be educated about contact with wildlife. Some wild animals may come close to human settlements and residential areas. As a general precaution, people should not approach or feed wild animals or touch or eat those that are orphaned, sick or found dead (including road kills). Instead, they should contact local wildlife authorities or a wildlife health professional.
It is also crucial to safely dispose of uneaten food, masks, tissues, and any other human waste to avoid attracting wildlife, especially to urban areas and, if possible, keep domestic animals away from wildlife and their droppings.
We furthermore encourage countries’ national animal and human health services to adopt the following measures:
Encourage collaboration between national veterinary services and national wildlife authorities, whose partnership is key to promoting animal health and safeguarding human and environmental health.
Promote monitoring of wildlife and encourage sampling of wild animals known to be potentially susceptible to SARS-CoV-2.
Share all genetic sequence data from animal surveillance studies through publicly available databases.
Report confirmed animal cases of SARS-CoV-2 to the OIE through the World Animal Health Information System (OIE-WAHIS).
Craft messages about SARS-CoV-2 in animals with care so that inaccurate public perceptions do not negatively impact conservation efforts. No animal found to be infected with SARS-CoV-2 should be abandoned, rejected, or killed without providing justification from a country- or event-specific risk assessment.
Suspend the sale of captured live wild mammals in food markets as an emergency measure .
Our organizations emphasize the importance of monitoring mammalian wildlife populations for SARS-CoV-2 infection, reporting results to National Veterinary Services (who report these findings to the OIE) and sharing genomic sequencing data on publicly available databases. Countries should also adopt precautions to reduce the risk of establishment of animal reservoirs and potential acceleration of virus evolution in novel hosts, which could lead to the emergence of new SARS-CoV-2 variants. Such measures will preserve the health of precious wildlife as well as humans.
We invite governments and other stakeholders to bring the contents of this joint statement to the attention of competent authorities and all parties concerned.
COVID IP – WIPO Report
New COVID-19 Research: Universities and Research Organizations Highly Active in Vaccine Patenting During Pandemic’s Early Days; China, U.S.-based Applicants Lead in Vaccine and Therapeutics Innovation
Mar 9, 2022 PR/2022/887
Universities and research organizations filed nearly as many patent applications as corporations for COVID-19 vaccines during the early months of the global pandemic, with China and U.S.-based innovators most actively patenting new anti-COVID19 vaccine and therapeutic technologies, according to a WIPO report on pandemic-era innovation trends released today.
“This report underlines that collaboration – across organizations, agencies, sectors and borders – is essential if we are to make meaningful progress in addressing the global challenges that we face,” said Mr. Tang, who was joined at an event launching the report by World Health Organization Director General Tedros Adhanom Ghebreyesus and World Trade Organization Director General Ngozi Okonjo-Iweala.
Among the report’s key findings
In the first 21 months of the pandemic, close to 5,300 patent applications relating to COVID-19 were filed across 49 patent offices.
This included nearly 1,500 filings related to therapeutics and over 400 filings related to vaccines.
For vaccine filings, universities and public research organizations accounted for 44 percent of the total, compared with 49 percent by companies. As one point of comparison, universities and public research organizations accounted for only 8 percent of total international patent applications filed with WIPO in 2021.
The top 10 applicant locations for vaccines were China, the U.S., the Russian Federation, the U.K., Republic of Korea, Germany, India, Austria, Switzerland and Australia.
China, the U.S. and India were the top origins for therapeutics. India and the Republic of Korea saw higher filing activity for therapeutics than vaccines.
Initial data from top patent offices showed comparatively quick grants for patent applications related to COVID-19, with innovators utilizing general fast-track avenues or specific COVID-19 measures designed to bring new products to the public quickly. By comparison, they were processed faster than patent applications in the field of chemistry and bioscience in the same period (January 2020 to September 2021).
The report also highlights how research organizations and universities worked together with private industry to help speed the development of life-saving COVID-19 vaccines and therapeutics, and confirms that accelerated innovation and vaccine development during the pandemic were possible thanks to research breakthroughs and technological advancements pre-pandemic.
WIPO Patent Landscape Report – COVID-19-related vaccines and therapeutics
Preliminary insights on related patenting activity during the pandemic
Author(s): World Intellectual Property Organization (WIPO) |
Publication year: 2022 :: 83 pages
Download: https://www.wipo.int/publications/en/details.jsp?id=4589
IP/TRIPS/WTO
Members updated on high-level talks aimed at finding convergence on IP COVID-19 response
World Trade Organisation – 10 March 2022
WTO members were updated on the high-level political consultations taking place to try to find a landing zone on the intellectual property-related aspects of a WTO COVID-19 response. At a meeting of the Council for Trade-Related Aspects of Intellectual Property Rights (TRIPS) on 9-10 March, members agreed to keep open two related agenda items on the WTO response to allow the Council to be reconvened at short notice when and if convergence is within reach. The Council elected Ambassador Lansana Gberie (Sierra Leone) as its new chair.
[Excerpts]
Some of the members participating since December 2021 in the high-level talks – the European Union, India, South Africa and the United States – expressed cautious optimism about a possible outcome and asked for patience from the rest of the membership. These members said that the small-group discussions continue to take place in good faith, with the objective of finding a landing zone that delivers on the common purpose of ensuring equitable access to vaccines, therapeutics, and diagnostics.
They stressed that any framework that emerges from this process, under the coordination of WTO Director-General Ngozi Okonjo-Iweala and Deputy Director-General Anabel González, will be subject to consideration by the TRIPS Council and its full membership. They also underlined that achieving an effective outcome on this critical issue would serve the ultimate purpose of tackling the ongoing crisis as well as ensuring that the credibility of the WTO is restored.
Generally, members were supportive of the small-group consultations and encouraged those participating to regularly update the TRIPS Council. However, some members asked for greater transparency and inclusiveness in order to reach an outcome that represents the views of the whole membership. For these members, progress towards an outcome on an IP COVID-19 response cannot be expected if the TRIPS Council does not know the details of discussions that can result in taking forward discussions on the wider pandemic response.
The chair of the TRIPS Council, Ambassador Dagfinn Sørli of Norway, said that expectations and hopes remain, as the current high-level process may result in framing a platform upon which the membership at large may be able to build something that can result in a consensus-based solution. He added that all efforts should be made to keep the entire membership informed and involved in the deliberation of the items which are part of the Council’s agenda.
Members agreed to keep open in the agenda of the TRIPS Council the proposal for a waiver from certain provisions of the TRIPS Agreement for the prevention, containment and treatment of COVID-19 (IP/C/W/669/Rev.1) and the Draft General Council Declaration on the TRIPS Agreement and Public Heath in the Circumstances of a Pandemic (IP/C/W/681) so that the Council can be reconvened at short notice if substantial progress is made in the high-level talks…
…COVID-19 update
Members were updated on IP measures in the context of COVID-19. The basis for the discussions was once more the compilation “COVID-19: Measures Regarding Trade-Related Intellectual Property Rights“. The WTO webpage contains a non-exhaustive list of IP-related measures taken in the context of COVID-19, compiled by the Secretariat from official sources and verified by the members concerned.
At the request of members, the WTO Secretariat provided an interim update on the number of voluntary licence agreements regarding COVID-19 vaccine production over time and on the projected and observed volume of vaccine dose production under these agreements.
The Secretariat also introduced the Annual Report on Notifications and other information flows (IP/C/W/687/Rev.1), which provides analysis and trends in members’ fulfilment of the TRIPS transparency obligations, including detailed member-by-member information on notifications and other TRIPS submissions…
…To date, 134 members have accepted the TRIPS Amendment, which entered into force on 23 January 2017, securing for developing countries a legal pathway to access affordable medicines under WTO rules. The chair said that, together with the Director-General, he had sent letters to the remaining 30 members encouraging them to expedite action in good time before the current deadline for acceptance, which was extended until 31 December 2021…
::::::
Coronavirus [COVID-19] – WHO
Public Health Emergency of International Concern (PHEIC)
https://www.who.int/emergencies/diseases/novel-coronavirus-2019
Weekly Epidemiological and Operational updates
Last update: 25 Feb 2022
Confirmed cases :: 452 201 564
Confirmed deaths :: 6 029 852
Vaccine doses administered: 10 704 043 684
::::::
Weekly epidemiological update on COVID-19 – 8 March 2022
Overview
Globally, during the week of 28 February through 6 March 2022, the number of new COVID-19 cases and deaths has continued to decline by 5% and 8% respectively, as compared to the previous week. Across the six WHO regions, over 10 million new cases and over 52 000 new deaths were reported. As of 6 March 2022, over 433 million confirmed cases and over 5.9 million deaths have been reported globally.
At the regional level, while the Western Pacific Region continue to report an increase (+46%) in the number of new weekly cases, all other regions reported decreases. The number of new weekly deaths increased in the Western Pacific (+29%) and the Eastern Mediterranean (+2%) Regions, while decreases were reported by the African Region (-39%), Europe Region (-15%), the Region of the Americas (-9%) and South-East Asia Region (-3%)…
::::::
WHO Director General Speeches [selected]
https://www.who.int/director-general/speeches
Selected
10 March 2022
Speech
WHO Director-General’s remarks at Launch of the WIPO Patent Landscape Report on COVID-19 – 10 March 2022
9 March 2022
Speech
WHO Director-General’s opening remarks at the media briefing on COVID-19 and Ukraine – 9 March 2022
8 March 2022
Speech
WHO Director-General’s keynote speech at the Global Pandemic Preparedness Summit
Status of COVID-19 Vaccines within WHO EUL/PQ evaluation process 02 March 2022
[Full scale view available at title link above]
[Updated on 02 March 2022]
::::::
COVID Vaccines/Therapeutics – Developer/Manufacturer Announcements
[Selected press releases/announcements from organizations from WHO EUL/PQ listing above and other organizations]
COVID Vaccines/Therapeutics – Developer/Manufacturer Announcements
[Selected press releases/announcements from organizations from WHO EUL/PQ listing above and other organizations]
AstraZeneca
Press Releases – No new digest announcements identified
Bharat Biotech
Press Releases – No new digest announcements identified
BioCubaFarma – Cuba
Últimas Noticias – Website not leading at inquiry
Biontech
Press Releases
Statement on Humanitarian Aid in Ukraine
7 March 2022
CanSinoBIO
News – [Website not responding at inquiry]
Clover Biopharmaceuticals – China
News – No new digest announcements identified
Curevac [Bayer Ag – Germany]
News
CureVac Establishes Fully-Owned Company Dedicated to Advancing The RNA Printer®
March 1, 2022
The RNA Printer® is CureVac’s integrated and automated manufacturing solution for RNA vaccines and therapeutics
CureVac RNA Printer GmbH to provide dedicated infrastructure to accelerate development and broaden application range of The RNA Printer®
Dr. Markus Bergmann appointed as General Manager for CureVac RNA Printer GmbH
Gamaleya National Center
Latest News and Events – See Russia below and under Ukraine above
IMBCAMS, China
Home – Website not responding at inquiry
Janssen/JNJ
Press Releases
Johnson & Johnson Announces Landmark Agreement to Enable its COVID-19 Vaccine to be Manufactured and Made Available by an African Company for People Living in Africa
United States
:: Aspen will manufacture and provide the Johnson & Johnson COVID-19 vaccine under its own brand name per the terms of agreement
:: Ensuring equitable, global access to COVID-19 vaccines has been a pillar of Johnson & Johnson’s response since the earliest days of the pandemic
:: The production of COVID-19 vaccines on multiple continents is critical to supporting an equitable response, controlling the pandemic, and improving long-term health security
NEW BRUNSWICK, NJ (March 8, 2022) – Johnson & Johnson (NYSE: JNJ) (the Company) today announced the completion of a landmark agreement between Janssen Pharmaceuticals, Inc., and manufacturer Aspen SA Operations (Pty) Ltd, based in South Africa, to enable the first COVID-19 vaccine to be manufactured and made available by an African company for people living in Africa, with the goal of increasing COVID-19 vaccination rates across the continent…
Moderna
Press Releases
March 10, 2022
Moderna Announces First Participant Dosed in Phase 2 Study of Omicron-Specific Bivalent Booster Candidate
March 7, 2022
Moderna Announces Its Global Public Health Strategy
:: Moderna announces commitment to advance vaccines targeting 15 pathogens identified as biggest public health risk by WHO and CEPI into clinical studies by 2025
:: Moderna launches mRNA Access, a new collaborative enabling researchers around the world to utilize Moderna’s mRNA technology platform to pursue research in their own labs on emerging and neglected infectious diseases
:: Moderna expands commitment to never enforce COVID-19 patents in the Gavi COVAX AMC for 92 low- and middle-income countries
:: Moderna has entered into a Memorandum of Understanding with the Government of the Republic of Kenya to establish its first mRNA manufacturing facility on the continent of Africa
Novavax
Press Releases
Novavax Announces Launch of Global Vaccine Education Programs
Mar 10, 2022
Novavax Statement on Availability of its COVID-19 Vaccine in New Zealand
Mar 08, 2022
Pfizer
Recent Press Releases
03.09.2022
Pfizer Initiates Phase 2/3 Study of Novel COVID-19 Oral Treatment in Pediatric Participants
Sanofi Pasteur
Press Releases – No new digest announcements identified
Serum Institute of India
NEWS & ANNOUNCEMENTS – No new digest announcements identified
Shifa Pharmed [Iran]
http://shafapharmed.com/
No news page identified.
Sinopharm/WIBPBIBP
News – No new digest announcements identified
Sinovac
Press Releases – No new digest announcements identified
Vector State Research Centre of Viralogy and Biotechnology
Home – No new digest announcements identified
Zhifei Longcom, China
[Anhui Zhifei Longcom Biologic Pharmacy Co., Ltd.]
[No website identified]
::::::
GSK
Press releases for media – No new digest announcements identified
Merck
News releases – No new digest announcements identified
Novartis
News – No new digest announcements identified
SK Biosciences
Press releases – No new digest announcements identified
Valneva
Press Releases
March 11, 2022
Valneva Provides Regulatory Update on its COVID-19 Vaccine Candidate
March 8, 2022
Valneva Successfully Completes Pivotal Phase 3 Trial of Single-Shot Chikungunya Vaccine Candidate
UNICEF COVID-19 Vaccine Market Dashboard :: Agreements Table Accessed 12 Mar 2022
An overview of information collected from publicly announced bilateral and multilateral supply agreements [no new agreements since 10/22/2021 reported]
COVID-19 Global Targets and Progress Tracker – IMF
The COVID-19 Global Targets and Progress Tracker presents a consolidated view of the progress towards global COVID-19 targets, barriers in access to COVID-19 tools, and delivery of donor pledges. The global targets presented in the Tracker are based on an alignment of the targets identified in the IMF Pandemic Proposal, ACT-A Strategic Plan & Budget, and the US-hosted Global C19 Summit, and as such have been reaffirmed by multilateral institutions and global leaders. We will continue to enhance the tracker as we improve our data collection efforts.
Global Dashboard on COVID-19 Vaccine Equity
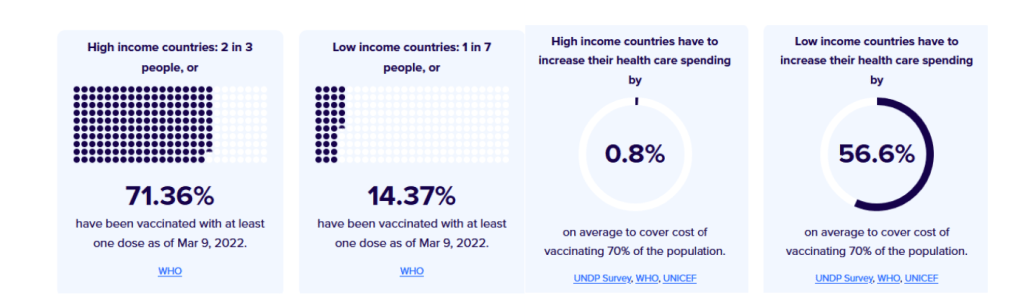
The Dashboard is a joint initiative of UNDP, WHO and the University of Oxford with cooperation across the UN system, anchored in the SDG 3 Global Action Plan for Healthy Lives and Well-being for All.
Dashboard on Vaccine Equity [accessed 12 Mar 2022]: https://data.undp.org/vaccine-equity/
See also visualization on Vaccine Access and Vaccine Affordability
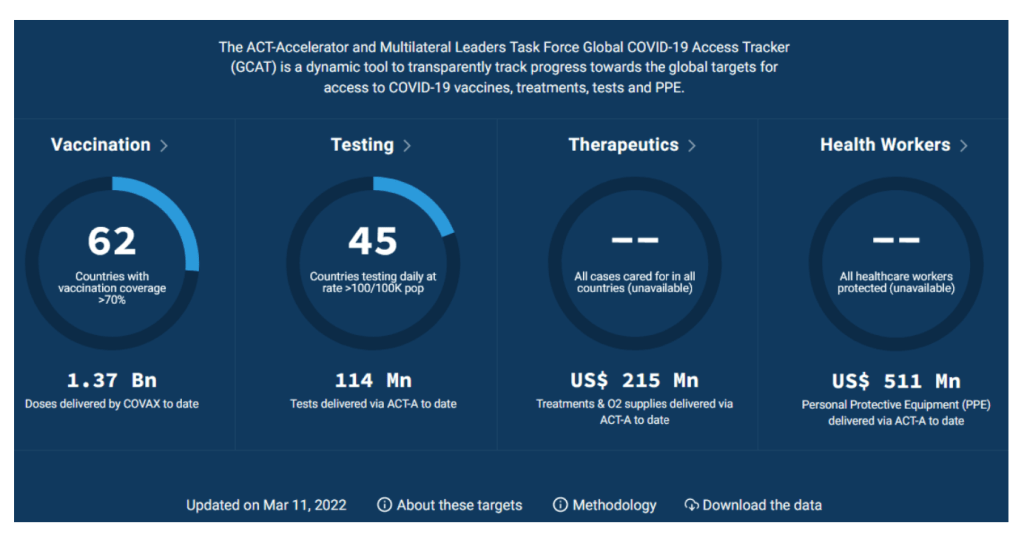
Global COVID-19 Access Tracker
https://www.covid19globaltracker.org/
Duke – Launch and Scale Speedometer
The Race for Global COVID-19 Vaccine Equity
A flurry of nearly 200 COVID-19 vaccine candidates are moving forward through the development and clinical trials processes at unprecedented speed; more than ten candidates are already in Phase 3 large-scale trials and several have received emergency or limited authorization. Our team has aggregated and analyzed publicly available data to track the flow of procurement and manufacturing and better understand global equity challenges. We developed a data framework of relevant variables and conducted desk research of publicly available information to identify COVID vaccine candidates and status, deals and ongoing negotiations for procurement and manufacturing, COVID burden by country, and allocation and distribution plans. We have also conducted interviews with public officials in key countries to better understand the context and challenges facing vaccine allocation and distribution
[accessed 24 July 2021]
See our COVID Vaccine Purchases research
See our COVID Vaccine Manufacturing research
See our COVID Vaccine Donations & Exports research
COVID Vaccines – OCHA:: HDX
COVID-19 Data Explorer: Global Humanitarian Operations
COVID-19 Vaccine Roll-out
12 Mar 2022 | COVAX (WHO,GAVI,CEPI), UNDESA, Press Reports | DATA
Global COVID-19 Figures: 4.52M total confirmed cases; 6.0M total confirmed deaths
Global vaccines administered: 10.9B
Number of Countries: 28
COVAX Allocations Round 4-9 (Number of Doses): 170M
COVAX Delivered (Number of Doses): 260M
Other Delivered (Number of Doses): 250M
Total Delivered (Number of Doses): 520M
Total Administered (Number of Doses): 350M